What are Telomeres
and Telomerase?
http://www4.utsouthwestern.edu/cellbio/shay-wright/intro/facts/sw_facts.html
and Telomerase?
http://www4.utsouthwestern.edu/cellbio/shay-wright/intro/facts/sw_facts.html
What are telomeres and telomerase?
To better understand telomeres and telomerase, let's first review some basic principles of biology and genetics. The human body is an organism formed by adding many organ systems together. Those organ systems are made of individual organs. Each organ contains tissues designed for specific functions like absorption and secretion.
Tissues are made of cells that have joined together to perform those special functions. Each cell is then made of smaller components called organelles, one of which is called the nucleus. The nucleus contains structures called chromosomes that are actually "packages" of all the genetic information that is passed from parents to their children.
The genetic information, or "genes", are really just a series of bases called Adenine (A), Guanine (G), Cytosine (C), and Thymine (T). These base pairs make up our cellular alphabet and create the sequences, or instructions needed to form our bodies. In order to grow and age, our bodies must duplicate their cells. This process is called mitosis. Mitosis is a process that allows one "parent" cell to divide into two new "daughter" cells.
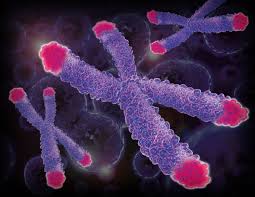
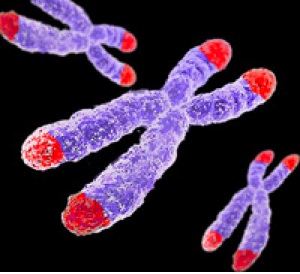
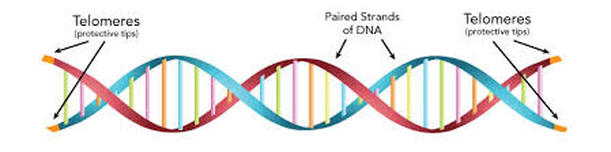
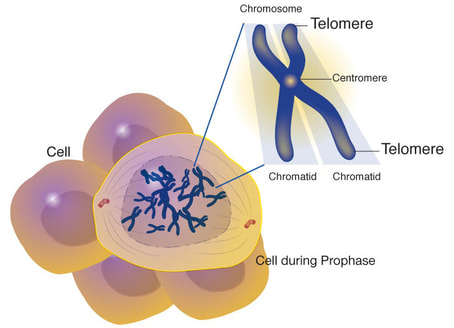
During mitosis, cells make copies of their genetic material. Half of the genetic material goes to each new daughter cell. To make sure that information is successfully passed from one generation to the next, each chromosome has a special protective cap called a telomere located at the end of its "arms". Telomeres are controlled by the presence of the enzyme telomerase. Now that we have covered some basics, let's explore telomeres, telomerase, and their importance to you! (view animation)
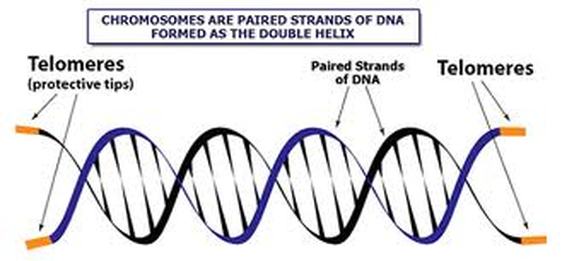
A telomere is a repeating DNA sequence (for example, TTAGGG) at the end of the body's chromosomes. The telomere can reach a length of 15,000 base pairs. Telomeres function by preventing chromosomes from losing base pair sequences at their ends. They also stop chromosomes from fusing to each other. However, each time a cell divides, some of the telomere is lost (usually 25-200 base pairs per division).
When the telomere becomes too short, the chromosome reaches a "critical length" and can no longer replicate. This means that a cell becomes "old" and dies by a process called apoptosis. Telomere activity is controlled by two mechanisms: erosion and addition. Erosion, as mentioned, occurs each time a cell divides. Addition is determined by the activity of telomerase. (view animation)
Telomerase, also called telomere terminal transferase, is an enzyme made of protein and RNA subunits that elongates chromosomes by adding TTAGGG sequences to the end of existing chromosomes. Telomerase is found in fetal tissues, adult germ cells, and also tumor cells. Telomerase activity is regulated during development and has a very low, almost undetectable activity in somatic (body) cells.
Because these somatic cells do not regularly use telomerase, they age. The result of aging cells is an aging body. If telomerase is activated in a cell, the cell will continue to grow and divide. This "immortal cell" theory is important in two areas of research: aging and cancer.
Cellular aging, or senescence, is the process by which a cell becomes old and dies. It is due to the shortening of chromosomal telomeres to the point that the chromosome reaches a critical length. Cellular aging is analogous to a wind up clock. If the clock stays wound, a cell becomes immortal and constantly produces new cells.
If the clock winds down, the cell stops producing new cells and dies. Our cells are constantly aging. Being able to make the body's cells live forever certainly creates some exciting possibilities. Telomerase research could therefore yield important discoveries related to the aging process.
Cancer cells are a type of malignant cell. The malignant cells multiply until they form a tumor that grows uncontrollably. Telomerase has been detected in human cancer cells and is found to be 10-20 times more active than in normal body cells. This provides a selective growth advantage to many types of tumors. If telomerase activity was to be turned off, then telomeres in cancer cells would shorten, just like they do in normal body cells.
This would prevent the cancer cells from dividing uncontrollably in their early stages of development. In the event that a tumor has already thoroughly developed, it may be removed and anti-telomerase therapy could be administered to prevent relapse. In essence, preventing telomerase from performing its function would change cancer cells from "immortal" to "mortal".(view animation)
Knowing what we have just learned about telomeres and telomerase, it can be said that scientists are on the verge of discovering many of telomerase's secrets. In the future, their research in the area of telomerase could uncover valuable information to combat aging, fight cancer, and even improve the quality of medical treatment in other areas such as skin grafts for burn victims, bone marrow transplants, and heart disease. Who knows how far this could go?
To better understand telomeres and telomerase, let's first review some basic principles of biology and genetics. The human body is an organism formed by adding many organ systems together. Those organ systems are made of individual organs. Each organ contains tissues designed for specific functions like absorption and secretion.
Tissues are made of cells that have joined together to perform those special functions. Each cell is then made of smaller components called organelles, one of which is called the nucleus. The nucleus contains structures called chromosomes that are actually "packages" of all the genetic information that is passed from parents to their children.
The genetic information, or "genes", are really just a series of bases called Adenine (A), Guanine (G), Cytosine (C), and Thymine (T). These base pairs make up our cellular alphabet and create the sequences, or instructions needed to form our bodies. In order to grow and age, our bodies must duplicate their cells. This process is called mitosis. Mitosis is a process that allows one "parent" cell to divide into two new "daughter" cells.
During mitosis, cells make copies of their genetic material. Half of the genetic material goes to each new daughter cell. To make sure that information is successfully passed from one generation to the next, each chromosome has a special protective cap called a telomere located at the end of its "arms". Telomeres are controlled by the presence of the enzyme telomerase. Now that we have covered some basics, let's explore telomeres, telomerase, and their importance to you! (view animation)
A telomere is a repeating DNA sequence (for example, TTAGGG) at the end of the body's chromosomes. The telomere can reach a length of 15,000 base pairs. Telomeres function by preventing chromosomes from losing base pair sequences at their ends. They also stop chromosomes from fusing to each other. However, each time a cell divides, some of the telomere is lost (usually 25-200 base pairs per division).
When the telomere becomes too short, the chromosome reaches a "critical length" and can no longer replicate. This means that a cell becomes "old" and dies by a process called apoptosis. Telomere activity is controlled by two mechanisms: erosion and addition. Erosion, as mentioned, occurs each time a cell divides. Addition is determined by the activity of telomerase. (view animation)
Telomerase, also called telomere terminal transferase, is an enzyme made of protein and RNA subunits that elongates chromosomes by adding TTAGGG sequences to the end of existing chromosomes. Telomerase is found in fetal tissues, adult germ cells, and also tumor cells. Telomerase activity is regulated during development and has a very low, almost undetectable activity in somatic (body) cells.
Because these somatic cells do not regularly use telomerase, they age. The result of aging cells is an aging body. If telomerase is activated in a cell, the cell will continue to grow and divide. This "immortal cell" theory is important in two areas of research: aging and cancer.
Cellular aging, or senescence, is the process by which a cell becomes old and dies. It is due to the shortening of chromosomal telomeres to the point that the chromosome reaches a critical length. Cellular aging is analogous to a wind up clock. If the clock stays wound, a cell becomes immortal and constantly produces new cells.
If the clock winds down, the cell stops producing new cells and dies. Our cells are constantly aging. Being able to make the body's cells live forever certainly creates some exciting possibilities. Telomerase research could therefore yield important discoveries related to the aging process.
Cancer cells are a type of malignant cell. The malignant cells multiply until they form a tumor that grows uncontrollably. Telomerase has been detected in human cancer cells and is found to be 10-20 times more active than in normal body cells. This provides a selective growth advantage to many types of tumors. If telomerase activity was to be turned off, then telomeres in cancer cells would shorten, just like they do in normal body cells.
This would prevent the cancer cells from dividing uncontrollably in their early stages of development. In the event that a tumor has already thoroughly developed, it may be removed and anti-telomerase therapy could be administered to prevent relapse. In essence, preventing telomerase from performing its function would change cancer cells from "immortal" to "mortal".(view animation)
Knowing what we have just learned about telomeres and telomerase, it can be said that scientists are on the verge of discovering many of telomerase's secrets. In the future, their research in the area of telomerase could uncover valuable information to combat aging, fight cancer, and even improve the quality of medical treatment in other areas such as skin grafts for burn victims, bone marrow transplants, and heart disease. Who knows how far this could go?
Stunning Information on Anti Aging
An Inexpensive Way to Reverse our Age
An Inexpensive Way to Reverse our Age
Jim Green in 2013 Jim Green in 2007
Who is Jim Green?
A one-man experiment in radical anti-aging
One reason that there is still so much uncertainty in anti-aging medicine is that we can’t do experiments on humans the way we do on mice. So we are grateful for a few human guinea pigs who put their own bodies on the line as early adopters. There is the Caloric Restriction Society, people who are losing weight in the hope of gaining years. There is an on-line community of people experimenting with Buckyballs in olive oil (this may sound like a joke if you haven’t read about it before).
And then there is Jim Green of Wichita, KS. Since 2007, Jim has been pursuing experimental strategies and claims to have set his body clock back by 15 to 20 years already. The core of his program is telomerase activation herbs, particularly astragalus, taken in much larger quantities than the label recommends. Jim has quite a story to tell, and this week I have interviewed him, an exclusive for ScienceBlog.
(You can read more about Jim’s career here and his anti-aging program here.)
What gave you the idea that growing younger was possible?
According to the telomere position effect data I saw early on back in 2007, telomere length impacts youthful gene expression, and gene expression is more youthful the longer the telomeres are. Furthermore, since 1998 it has been realized that senescent cells can often be restored to the youthful phenotype by transfecting them with a virus that improves hTERT transcription and boosts levels of telomerasee in the cell. In 1998, Michael Fossel published an article on this in the Journal of Anti-Aging Medicine (now Rejuvenation Research). [Another Fossel article -JJM] So there were definite grounds for optimism that lengthening telomeres with telomerase could result in rejuvenation transformations.
On the other hand, as telomeres got shorter, patterns of gene expression became more elderly and the likelihood of the onset of diseases of old ageincreased, according to many scientific studies. For instance, microglial cells in the brain that clean up amyloid beta plaque leading to Alzheimer’s disease fail when they become senescent. If senescence in the microglial cells (derived from hematopoietic stem cells) could be prevented, then Alzheimer’s disease due to the onset of senility might also be prevented.
Similarly, when the lining of the vascular endothelium becomes senescent, it becomes more adhesive to monocytes and more likely to develop atherosclerotic plaqueleading to attacks or strokes. So taking small molecule telomerase activators effective at increasing the telomere length of components of the blood was a very good bet for effective life extension. Similarly, when dermal fibroblasts go senescent, they begin to attack the extracellular matrix, producing wrinkles.
This does not happen at once to all of the fibroblasts, but gradually in a way that produces more and more defects in the extracellular matrix behind wrinkles. Thus a telomerase activator effective on dermal fibroblasts should prevent observable signs of old age such as wrinkles.
When did you start your program? What was your program in the beginning?
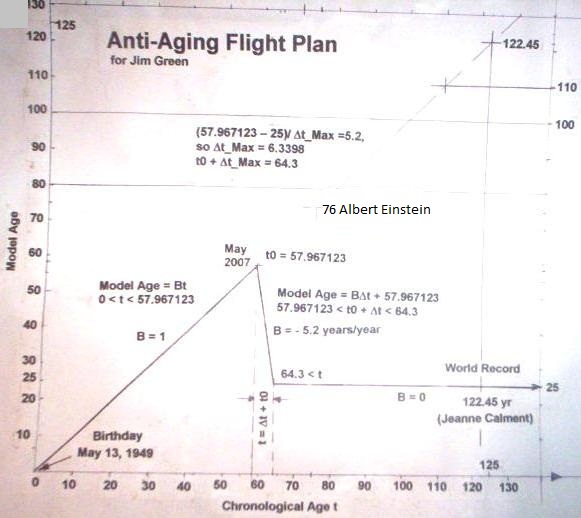
I started taking telomerase activators on May 1, 2007, after reading material on TA-65 from TA Sciences. I also had an exchange with Greta Blackburn of TA Sciences in which she emailed me promising results obtained by using TA-41, an astragalus extract which they believed would be similar in effect to TA-65.
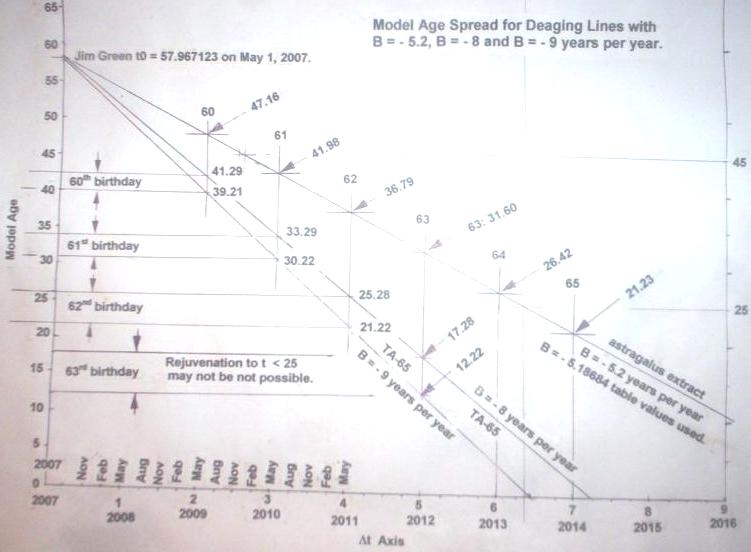
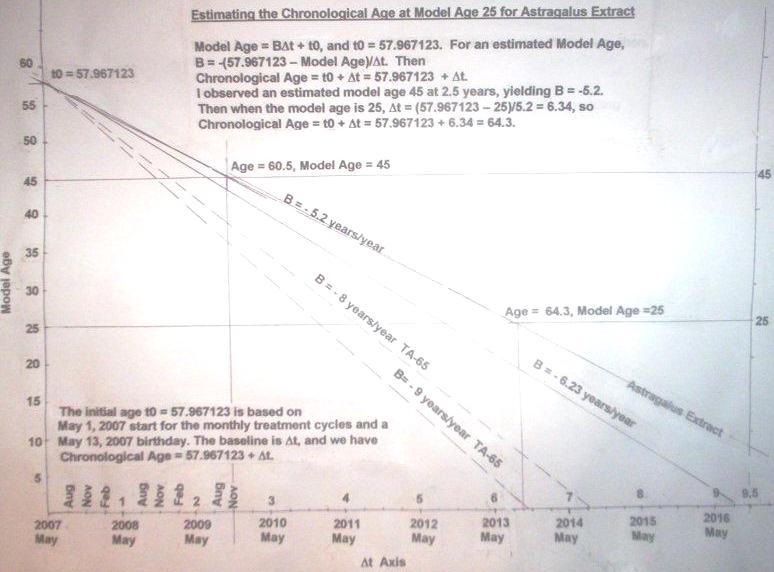
At first I expected to be able to set back my telomere clock by up to 8 or 9 years per year, but subsequently revised this to about 5.1 years per year.
The astragalus extract actually finally turned out to work rather better, in fact, than TA-65.
I was also reading Life Extension Magazine since 2004, so I was familiar with supplements recommended by LEF to prevent diseases of old age, such asacetyl L-carnitine with alpha lipoic acid for mitochondria, L-arginine as a nitric oxide booster for rejuvenating the vascular endothelium, anticancer supplements such as garlic and ginger and turmeric, and so forth.
At first I took astragalus extracts such a Solaray Astragalus Extract, and tried GAIA Herbs astragalus extracts. Later, I got the idea of using colostrum skin creams for telomerase activation. However I seem to have used astragalus extracts both orally and on my skin in the beginning. This was done for two weeks before switching to a telomerase-inhibiting anticancer phase of treatment for the next two weeks, when I took medicine like curcumin, which is anticancer. If my telomerase activators tended at all to encourage cancer, I was going to squelch that every monthZin November 2009 I presented a short description of my program here For 2 1/2 years, I had relied primarily on astragalus extract for telomerase activation. However, I also got 5 grams of arginine per day to improve my nitric oxide levels and improve the length of endothelial progenitor cell telomeres.
By June of 2009 I concluded after studies of Vida Institute literature that 33 grams/day of astragalus root would serve as well or better than the 5 mg/day of astragalosides from astragalus extract that I computed I was taking. So I switched from pure astragalus extract like TA-41, which worked fine according to Greta Blackburn at TA Sciences, to astragalus root in capsules from NOW FOODS. However, I was still applying astragalus extract directly to my scalp.
[Editor’s note: Astragalus is a woody root which can be found in Chinese groceries for about $10/lb. Traditional use would be to boil strips of astraglus to make a tea. -JJM]
What is your supplements regimen at present? Is it still evolving?
I have posted a short summary of my present program and photo news here. It is still evolving, and by now I have 173 items at least on my list of telomerase activators that I am exploring. The numbers of the activators chronicle the order in which I found them in the literature, or were tipped off to them by other investigators, except for sub-items, which I have sometimes found more frequently.
I have continued a four-week cycle featuring telomerase activators for two weeks followed by anticancer telomerase inhibitors for two weeks. I do things that permit cells to recover from the senescent state that are a little unusual now: I use folic acid in my colostrum solution with an amphipathic alcohol to improve absorption (rubbing alcohol = isopropyl alcohol). [Isopropyl alcohol is moderately toxic. - JJM] I take carrots and exercise to inhibit P16INK4A, which can stop senescent cell recovery.
I have many ideas about medicine I would like to include, such as tocotrienol-rich fraction. [These are chemical cousins of vitamin E derived from grains -JJM] For bodybuilding, I take whey protein and creatine monohydrate, although I am not attempting a championship program. I can more easily resemble a gymnast.
Besides supplements, what are you doing to stay young?
Exercise improves circulation in the brain, elevates nitric oxide levels that dialate vessels, and provides numerous growth factor telomerase activators that can be separately enhanced by various nutraceuticals. So I work out with weights, about 6 sets per muscle, using a split routine that takes 3 or 4 days. This might be dangerous to a person with too much atherosclerotic plaque, or someone with glycation stiffening in the veinous system from high levels of sugar from ice cream. However, it tends to lower triglycerides and improve the lipid profile, and also to improve confidence and mood.
I take a slice of Swiss cheese often to get a higher vitamin K2 level to avoid calcification of the arterial system and aortic stenosis.
In what ways do you feel younger than 7 years ago?
May 2007 was my starting date, and we are now approaching May 2014. I would say that my confidence has improved that we can finally master aging, although there is still a fair amount of suspense involved. My physical condition oscillates somewhat, and is subject to minor perturbations. I work to get leaner in the Spring, like most people, and my self-image improves then. I look better in photographs, although I invariably puff up a bit in the Winter. In the Summer I can do pull-ups on outside chinning bars, and I get more V-shaped.
In general, I seem to look somewhat better to myself in photographs an I did 7 years ago. Last Summer I did 130 pushups, which was an all-time record for me. I am hoping that it will go back up to that this summer.
I notice you pay attention to appearance as well as health. Do you think there’s a connection between anti-aging skin treatments and longevity? Or a psychological effect whereby looking younger helps you to feel younger and programs your mind to live longer?
I could dress better to improve my appearance, but my funds vanish into supplements. Wrinkles are definitely symptomatic of the formation of senescent cells which begin to excrete factors that attack the extracellular matrix and weaken it. A weaker extracellular matrix leads to more than cosmetic problems, eventually, and less resistance available to UV radiation from the sun.
So keeping the skin healthy and young is very important for survival as well as for image. Skin cancer kills. Thin skin and failing veil cells around veins can produce bleeding, finally, perhaps even dangerous internal bleeding.
Of course, looking younger creates a sense of optimism about what may be possible, and helps reinforce a positive attitude.
What evidence do you have that your body is really younger?
Unfortunately, I was unable to do measurements of my blood component telomere lengths because of funds could not be mobilized in time. Therefore, about all I have is my collection of photographs online. There are many measures of aging and senescence that may be applied to the problem of measuring the effect when funds are available. See http://www.greenray4ever.com/lifexmortality.html.
* I wish I could say, “Testing experimental drugs on unsuspecting humans is off the table, out of the question, never done.” The truth is that it is not as exceptional as we might wish. And the institutions that resort to surreptitious experimentation are usually up to no good. [MK-Ultra, Nazis, antibiotics to children, Tuskegee syphilis experiments]
A one-man experiment in radical anti-aging
One reason that there is still so much uncertainty in anti-aging medicine is that we can’t do experiments on humans the way we do on mice. So we are grateful for a few human guinea pigs who put their own bodies on the line as early adopters. There is the Caloric Restriction Society, people who are losing weight in the hope of gaining years. There is an on-line community of people experimenting with Buckyballs in olive oil (this may sound like a joke if you haven’t read about it before).
And then there is Jim Green of Wichita, KS. Since 2007, Jim has been pursuing experimental strategies and claims to have set his body clock back by 15 to 20 years already. The core of his program is telomerase activation herbs, particularly astragalus, taken in much larger quantities than the label recommends. Jim has quite a story to tell, and this week I have interviewed him, an exclusive for ScienceBlog.
(You can read more about Jim’s career here and his anti-aging program here.)
What gave you the idea that growing younger was possible?
According to the telomere position effect data I saw early on back in 2007, telomere length impacts youthful gene expression, and gene expression is more youthful the longer the telomeres are. Furthermore, since 1998 it has been realized that senescent cells can often be restored to the youthful phenotype by transfecting them with a virus that improves hTERT transcription and boosts levels of telomerasee in the cell. In 1998, Michael Fossel published an article on this in the Journal of Anti-Aging Medicine (now Rejuvenation Research). [Another Fossel article -JJM] So there were definite grounds for optimism that lengthening telomeres with telomerase could result in rejuvenation transformations.
On the other hand, as telomeres got shorter, patterns of gene expression became more elderly and the likelihood of the onset of diseases of old ageincreased, according to many scientific studies. For instance, microglial cells in the brain that clean up amyloid beta plaque leading to Alzheimer’s disease fail when they become senescent. If senescence in the microglial cells (derived from hematopoietic stem cells) could be prevented, then Alzheimer’s disease due to the onset of senility might also be prevented.
Similarly, when the lining of the vascular endothelium becomes senescent, it becomes more adhesive to monocytes and more likely to develop atherosclerotic plaqueleading to attacks or strokes. So taking small molecule telomerase activators effective at increasing the telomere length of components of the blood was a very good bet for effective life extension. Similarly, when dermal fibroblasts go senescent, they begin to attack the extracellular matrix, producing wrinkles.
This does not happen at once to all of the fibroblasts, but gradually in a way that produces more and more defects in the extracellular matrix behind wrinkles. Thus a telomerase activator effective on dermal fibroblasts should prevent observable signs of old age such as wrinkles.
When did you start your program? What was your program in the beginning?
I started taking telomerase activators on May 1, 2007, after reading material on TA-65 from TA Sciences. I also had an exchange with Greta Blackburn of TA Sciences in which she emailed me promising results obtained by using TA-41, an astragalus extract which they believed would be similar in effect to TA-65.
At first I expected to be able to set back my telomere clock by up to 8 or 9 years per year, but subsequently revised this to about 5.1 years per year.
The astragalus extract actually finally turned out to work rather better, in fact, than TA-65.
I was also reading Life Extension Magazine since 2004, so I was familiar with supplements recommended by LEF to prevent diseases of old age, such asacetyl L-carnitine with alpha lipoic acid for mitochondria, L-arginine as a nitric oxide booster for rejuvenating the vascular endothelium, anticancer supplements such as garlic and ginger and turmeric, and so forth.
At first I took astragalus extracts such a Solaray Astragalus Extract, and tried GAIA Herbs astragalus extracts. Later, I got the idea of using colostrum skin creams for telomerase activation. However I seem to have used astragalus extracts both orally and on my skin in the beginning. This was done for two weeks before switching to a telomerase-inhibiting anticancer phase of treatment for the next two weeks, when I took medicine like curcumin, which is anticancer. If my telomerase activators tended at all to encourage cancer, I was going to squelch that every monthZin November 2009 I presented a short description of my program here For 2 1/2 years, I had relied primarily on astragalus extract for telomerase activation. However, I also got 5 grams of arginine per day to improve my nitric oxide levels and improve the length of endothelial progenitor cell telomeres.
By June of 2009 I concluded after studies of Vida Institute literature that 33 grams/day of astragalus root would serve as well or better than the 5 mg/day of astragalosides from astragalus extract that I computed I was taking. So I switched from pure astragalus extract like TA-41, which worked fine according to Greta Blackburn at TA Sciences, to astragalus root in capsules from NOW FOODS. However, I was still applying astragalus extract directly to my scalp.
[Editor’s note: Astragalus is a woody root which can be found in Chinese groceries for about $10/lb. Traditional use would be to boil strips of astraglus to make a tea. -JJM]
What is your supplements regimen at present? Is it still evolving?
I have posted a short summary of my present program and photo news here. It is still evolving, and by now I have 173 items at least on my list of telomerase activators that I am exploring. The numbers of the activators chronicle the order in which I found them in the literature, or were tipped off to them by other investigators, except for sub-items, which I have sometimes found more frequently.
I have continued a four-week cycle featuring telomerase activators for two weeks followed by anticancer telomerase inhibitors for two weeks. I do things that permit cells to recover from the senescent state that are a little unusual now: I use folic acid in my colostrum solution with an amphipathic alcohol to improve absorption (rubbing alcohol = isopropyl alcohol). [Isopropyl alcohol is moderately toxic. - JJM] I take carrots and exercise to inhibit P16INK4A, which can stop senescent cell recovery.
I have many ideas about medicine I would like to include, such as tocotrienol-rich fraction. [These are chemical cousins of vitamin E derived from grains -JJM] For bodybuilding, I take whey protein and creatine monohydrate, although I am not attempting a championship program. I can more easily resemble a gymnast.
Besides supplements, what are you doing to stay young?
Exercise improves circulation in the brain, elevates nitric oxide levels that dialate vessels, and provides numerous growth factor telomerase activators that can be separately enhanced by various nutraceuticals. So I work out with weights, about 6 sets per muscle, using a split routine that takes 3 or 4 days. This might be dangerous to a person with too much atherosclerotic plaque, or someone with glycation stiffening in the veinous system from high levels of sugar from ice cream. However, it tends to lower triglycerides and improve the lipid profile, and also to improve confidence and mood.
I take a slice of Swiss cheese often to get a higher vitamin K2 level to avoid calcification of the arterial system and aortic stenosis.
In what ways do you feel younger than 7 years ago?
May 2007 was my starting date, and we are now approaching May 2014. I would say that my confidence has improved that we can finally master aging, although there is still a fair amount of suspense involved. My physical condition oscillates somewhat, and is subject to minor perturbations. I work to get leaner in the Spring, like most people, and my self-image improves then. I look better in photographs, although I invariably puff up a bit in the Winter. In the Summer I can do pull-ups on outside chinning bars, and I get more V-shaped.
In general, I seem to look somewhat better to myself in photographs an I did 7 years ago. Last Summer I did 130 pushups, which was an all-time record for me. I am hoping that it will go back up to that this summer.
I notice you pay attention to appearance as well as health. Do you think there’s a connection between anti-aging skin treatments and longevity? Or a psychological effect whereby looking younger helps you to feel younger and programs your mind to live longer?
I could dress better to improve my appearance, but my funds vanish into supplements. Wrinkles are definitely symptomatic of the formation of senescent cells which begin to excrete factors that attack the extracellular matrix and weaken it. A weaker extracellular matrix leads to more than cosmetic problems, eventually, and less resistance available to UV radiation from the sun.
So keeping the skin healthy and young is very important for survival as well as for image. Skin cancer kills. Thin skin and failing veil cells around veins can produce bleeding, finally, perhaps even dangerous internal bleeding.
Of course, looking younger creates a sense of optimism about what may be possible, and helps reinforce a positive attitude.
What evidence do you have that your body is really younger?
Unfortunately, I was unable to do measurements of my blood component telomere lengths because of funds could not be mobilized in time. Therefore, about all I have is my collection of photographs online. There are many measures of aging and senescence that may be applied to the problem of measuring the effect when funds are available. See http://www.greenray4ever.com/lifexmortality.html.
* I wish I could say, “Testing experimental drugs on unsuspecting humans is off the table, out of the question, never done.” The truth is that it is not as exceptional as we might wish. And the institutions that resort to surreptitious experimentation are usually up to no good. [MK-Ultra, Nazis, antibiotics to children, Tuskegee syphilis experiments]

What is TA-65 ?
TA-65 ® is a patented, all natural, plant-based compound which can help maintain or rebuild telomeres, that diminish as people get older.
The length of a person’s telomeres is a good indicator of his or her overall health status; short telomeres have been associated with cellular aging and dysfunction.1,2 The real biological age of a person’s body may be more or less than their chronological age. Telomere length is considered a key marker in measuring a person’s biological age as opposed to their chronological age.
For a body to stay healthy, it is important to maintain telomere length. Having short telomeres can accelerate the natural aging process on a cellular level. / Click here to learn more about Telomere Biology
By activating an enzyme called telomerase, the TA-65® compound can help slow down and possibly reverse age and lifestyle-related telomere shortening. / Click here to learn more about Telomerase Activation
TA-65 ® is a patented, all natural, plant-based compound which can help maintain or rebuild telomeres, that diminish as people get older.
The length of a person’s telomeres is a good indicator of his or her overall health status; short telomeres have been associated with cellular aging and dysfunction.1,2 The real biological age of a person’s body may be more or less than their chronological age. Telomere length is considered a key marker in measuring a person’s biological age as opposed to their chronological age.
For a body to stay healthy, it is important to maintain telomere length. Having short telomeres can accelerate the natural aging process on a cellular level. / Click here to learn more about Telomere Biology
By activating an enzyme called telomerase, the TA-65® compound can help slow down and possibly reverse age and lifestyle-related telomere shortening. / Click here to learn more about Telomerase Activation
What Is Astragalus? / High in TA-65
Astragalus slows down the aging process.
Astragalus root has been used in traditional Chinese medicine for centuries as a restorative tonic; it is considered a sweet, warming herb with effects on many organs. It is used either alone or with other herbs to help with aging, improve energy, and stimulate the immune system during conditions such as the common cold, blood disorders, cancer and HIV/AIDS. It is also used as an adaptogen, which is meant to increase general resistance to stress and disease, and normalize disturbances in your body’s ability to balance itself.
How Does It Work?
There's a special chemical in astragalus that actually slows down the aging process right where it happens, inside of our cells, where the blueprint of our cells resides.
Recent research has shown that this special chemical derived from astragalus can "turn on" an enzyme called telomerase (hTERT). Telomerase acts to maintain or lengthen telomeres, which extend the lifespan of your DNA.
If you imagine DNA as a shoelace, telomeres are the plastic aglets at each end. It serves as a protector for your DNA because it keeps it from fraying or damaging. As you age, your telomeres shorten due to wear and tear, which gives your cells an expiration date. However, telomerase helps to preserve telomeres by making them longer. Telemerase is usually "off" in adult cells, except in immune cells, in egg and sperm cells, and in malignant cells, like those found in cancer.
The length of your telomeres is important: Researchers have discovered correlations between telomere length and susceptibility to certain aging-related diseases, like cardiovascular diseases (heart attacks, atherosclerosis, and strokes), diabetes, and cancer.
Astragalus slows down the aging process.
Astragalus root has been used in traditional Chinese medicine for centuries as a restorative tonic; it is considered a sweet, warming herb with effects on many organs. It is used either alone or with other herbs to help with aging, improve energy, and stimulate the immune system during conditions such as the common cold, blood disorders, cancer and HIV/AIDS. It is also used as an adaptogen, which is meant to increase general resistance to stress and disease, and normalize disturbances in your body’s ability to balance itself.
How Does It Work?
There's a special chemical in astragalus that actually slows down the aging process right where it happens, inside of our cells, where the blueprint of our cells resides.
Recent research has shown that this special chemical derived from astragalus can "turn on" an enzyme called telomerase (hTERT). Telomerase acts to maintain or lengthen telomeres, which extend the lifespan of your DNA.
If you imagine DNA as a shoelace, telomeres are the plastic aglets at each end. It serves as a protector for your DNA because it keeps it from fraying or damaging. As you age, your telomeres shorten due to wear and tear, which gives your cells an expiration date. However, telomerase helps to preserve telomeres by making them longer. Telemerase is usually "off" in adult cells, except in immune cells, in egg and sperm cells, and in malignant cells, like those found in cancer.
The length of your telomeres is important: Researchers have discovered correlations between telomere length and susceptibility to certain aging-related diseases, like cardiovascular diseases (heart attacks, atherosclerosis, and strokes), diabetes, and cancer.
Fenugreek Seed
Organic / TA-65
https://www.swansonvitamins.com/starwest-botanicals-fenugreek-seed-organic-1-lb-pkg
Organic / TA-65
https://www.swansonvitamins.com/starwest-botanicals-fenugreek-seed-organic-1-lb-pkg
Fenugreek is an herb that is normally seen growing in the Mediterranean. It’s also used to take care of many different health issues in Egypt, Greece, Italy, and South Asia, while the leaves and seeds are chiefly used as a culinary spice. Some of the active components in fenugreek are alkaloids, lys and L – tryptophan, in addition to steroidal saponins (tigogenin, diosgenin, neotigogenin and yamogenin).
13 Ways it Helps Us
1) Anti-Inflammatory Properties
Many herbalists recommend fenugreek for lesions, healing rashes, and boils for its anti-inflammatory properties. Distribute the paste on the affected region. Commission E, a German authorities group that assesses the safety and effectiveness of herbs, has authorized it to treat gastritis. The plant’s seeds include mucilage, a gummy substance that coats the lining of the bowel, soothing gastrointestinal inflammation.
2) Lowers Cholesterol Levels
Fenugreek seeds aid in lowering LDL cholesterol within the blood and raises HDL within the body. They help in lowering cholesterol and triglycerides. It raises the creation of bile acids. The liver makes use of cholesterol for the creation of bile acids and subsequently reduces cholesterol. Saponin and galactomannan also assists in cholesterol absorbtion.
3) Helps Against Diabetes
Fenugreek happens to be one of the healthiest foods that a person can have if they have diabetes. It’s been found to reduce the blood glucose level within the body and raise the tolerance of glucose.
4) Helps With Digestion
Fenugreek helps to improve digestion. It releases mucilage, which creates a protective layer for gut and bowels, and lowers indigestion and gastric problems. Fenugreek has been discovered to stop constipation and increases bowel movement.
5) Heartburn and Acid Reflux
As I said, fenugreek seeds include lots of mucilage, which helps with soothing an inflamed gastrointestinal tract by being placed on the lining of the intestine and stomach. In order to utilize this as an effective treatment against heartburn, just scatter 1 teaspoon of fenugreek seeds on your food. Another alternative would be to consume them with water or juice and take one teaspoonful of seeds prior to eating.
6) Helps With Weight Loss
Fenugreek seeds soaked in water immediately can be consumed in the morning on an empty stomach to burn off extra fat within your body. Fenugreek seeds are full of fiber, which swells in the body and makes you feel less hungry. Overeating can be prevented by adopting this treatment in the morning and half an hour before dinner.
7) Makes Skin Healthy
Because fenugreek is anti-inflammatory it is used to heal eczema, burns and boils. Grounded fenugreek seeds along with water can be immediately placed on the acne and zit marks to help get rid of them. Fenugreek mixed with milk is used to help soften the skin. It also aids against harmful sun radiation.
8) Nourishes the Hair
Fenugreek seeds are beneficial in making hair grow healthy. Fenugreek seeds are a great source of protein and nicotinic acid, which assists in cutting thinning hair and baldness down. When applied it helps in reducing dandruff and functions like a conditioner, when you make a paste of the leaves.
9) Reduces Fever
Since the body is nourished by this herb, it is proven to help reduce fever when taken with honey and lemon. Some health food stores also sell teas that contain fenugreek, which may be utilized to help increase one’s immune system and break that fever.
10) Helps With Respiratory Conditions
Fenugreek seeds contain mucilage, a lubricating agent which could reduce redness and soothe irritated mucus membranes, making this herb helpful for treating respiratory conditions, including bronchitis and coughs.
11) Prevents Cancer
Colon cancer may be prevented by fenugreek consumption. The mucus membrane of the colon is protected through this, therefore reducing the possibilities of cancer.
12) For Women
Fenugreek is proven to raise milk production in lactating women. Fenugreek is advantageous for women while pregnant and is really a source of iron. Fenugreek tea is suggested for pregnant women as it reduces labour pain and improves uterine contraction. Fenugreek contains a substance called diogenin which behaves similar to estrogens. It reduces stress, dizziness, and sleeplessness and helps in cutting menopause symptoms. The herb is also said to cause breast enlargement in women.
13) For Men
Fenugreek is considered to be an aphrodisiac. It’s been discovered to raise testosterone in men. In addition, it’s anabolic and can help guys that are seeking to gain muscle. A high testosterone level helps in with keeping up one’s energy levels. It’s also efficient against the pain of a hernia and E.D.
Fenugreek Side Effects
While fenugreek is normally regarded as safe, there are reports of a few mild side effects. When you use this herb topically on your skin it’s important to look out for skin irritations and rashes.
Using fenugreek when pregnant isn’t recommended, because it really has the potential to cause labour. If you want to have it and are pregnant, you need to do so only after you meet and consult with your physician.
If you’re now taking any oral medicines, you must be sure to only use the herb at least 2 hrs before or after these drugs. That is essential since the fiber in fenugreek has the capability to get in the way of the absorption of oral medications because of its mucilaginous fiber.
Regardless of the recorded fenugreek advantages to human health, be certain to ask your physician or primary healthcare provider prior to taking fenugreek. You should discuss taking this with your primary care provider if you’re on any medicines, or if you’re taking some other medication or herbal supplements, because fenugreek can change blood glucose levels and blood clotting factors.
13 Ways it Helps Us
1) Anti-Inflammatory Properties
Many herbalists recommend fenugreek for lesions, healing rashes, and boils for its anti-inflammatory properties. Distribute the paste on the affected region. Commission E, a German authorities group that assesses the safety and effectiveness of herbs, has authorized it to treat gastritis. The plant’s seeds include mucilage, a gummy substance that coats the lining of the bowel, soothing gastrointestinal inflammation.
2) Lowers Cholesterol Levels
Fenugreek seeds aid in lowering LDL cholesterol within the blood and raises HDL within the body. They help in lowering cholesterol and triglycerides. It raises the creation of bile acids. The liver makes use of cholesterol for the creation of bile acids and subsequently reduces cholesterol. Saponin and galactomannan also assists in cholesterol absorbtion.
3) Helps Against Diabetes
Fenugreek happens to be one of the healthiest foods that a person can have if they have diabetes. It’s been found to reduce the blood glucose level within the body and raise the tolerance of glucose.
4) Helps With Digestion
Fenugreek helps to improve digestion. It releases mucilage, which creates a protective layer for gut and bowels, and lowers indigestion and gastric problems. Fenugreek has been discovered to stop constipation and increases bowel movement.
5) Heartburn and Acid Reflux
As I said, fenugreek seeds include lots of mucilage, which helps with soothing an inflamed gastrointestinal tract by being placed on the lining of the intestine and stomach. In order to utilize this as an effective treatment against heartburn, just scatter 1 teaspoon of fenugreek seeds on your food. Another alternative would be to consume them with water or juice and take one teaspoonful of seeds prior to eating.
6) Helps With Weight Loss
Fenugreek seeds soaked in water immediately can be consumed in the morning on an empty stomach to burn off extra fat within your body. Fenugreek seeds are full of fiber, which swells in the body and makes you feel less hungry. Overeating can be prevented by adopting this treatment in the morning and half an hour before dinner.
7) Makes Skin Healthy
Because fenugreek is anti-inflammatory it is used to heal eczema, burns and boils. Grounded fenugreek seeds along with water can be immediately placed on the acne and zit marks to help get rid of them. Fenugreek mixed with milk is used to help soften the skin. It also aids against harmful sun radiation.
8) Nourishes the Hair
Fenugreek seeds are beneficial in making hair grow healthy. Fenugreek seeds are a great source of protein and nicotinic acid, which assists in cutting thinning hair and baldness down. When applied it helps in reducing dandruff and functions like a conditioner, when you make a paste of the leaves.
9) Reduces Fever
Since the body is nourished by this herb, it is proven to help reduce fever when taken with honey and lemon. Some health food stores also sell teas that contain fenugreek, which may be utilized to help increase one’s immune system and break that fever.
10) Helps With Respiratory Conditions
Fenugreek seeds contain mucilage, a lubricating agent which could reduce redness and soothe irritated mucus membranes, making this herb helpful for treating respiratory conditions, including bronchitis and coughs.
11) Prevents Cancer
Colon cancer may be prevented by fenugreek consumption. The mucus membrane of the colon is protected through this, therefore reducing the possibilities of cancer.
12) For Women
Fenugreek is proven to raise milk production in lactating women. Fenugreek is advantageous for women while pregnant and is really a source of iron. Fenugreek tea is suggested for pregnant women as it reduces labour pain and improves uterine contraction. Fenugreek contains a substance called diogenin which behaves similar to estrogens. It reduces stress, dizziness, and sleeplessness and helps in cutting menopause symptoms. The herb is also said to cause breast enlargement in women.
13) For Men
Fenugreek is considered to be an aphrodisiac. It’s been discovered to raise testosterone in men. In addition, it’s anabolic and can help guys that are seeking to gain muscle. A high testosterone level helps in with keeping up one’s energy levels. It’s also efficient against the pain of a hernia and E.D.
Fenugreek Side Effects
While fenugreek is normally regarded as safe, there are reports of a few mild side effects. When you use this herb topically on your skin it’s important to look out for skin irritations and rashes.
Using fenugreek when pregnant isn’t recommended, because it really has the potential to cause labour. If you want to have it and are pregnant, you need to do so only after you meet and consult with your physician.
If you’re now taking any oral medicines, you must be sure to only use the herb at least 2 hrs before or after these drugs. That is essential since the fiber in fenugreek has the capability to get in the way of the absorption of oral medications because of its mucilaginous fiber.
Regardless of the recorded fenugreek advantages to human health, be certain to ask your physician or primary healthcare provider prior to taking fenugreek. You should discuss taking this with your primary care provider if you’re on any medicines, or if you’re taking some other medication or herbal supplements, because fenugreek can change blood glucose levels and blood clotting factors.
Black Cohosh (Cimicifuga Racemosa) / TA-65
Black Cohosh Benefits
http://www.herbwisdom.com/herb-black-cohosh.html#notes-side-effects
Black Cohosh has been used by Native Americans for more than two hundred years, after they discovered the root of the plant helped relieve menstrual cramps and symptoms of menopause. These days it is still used for menopausal symptoms such as hot flashes/flushes, irritability, mood swings and sleep disturbances. It is also used for PMS, menstrual irregularities, uterine spasms and has been indicated for reducing inflammation associated with osteoarthritis, rheumatoid arthritis and neuralgia.
Herbal researcher Dr. James Duke has this to say about Black Cohosh; "Black cohosh really should be better known in this country, especially with our aging population and the millions of women who are now facing menopause. Recognized for its mild sedative and anti-inflammatory activity, black cohosh can help with hot flashes and other symptoms associated with that dramatic change of life called menopause. It's also reported to have some estrogenic activity. Herbalist Steven Foster refers to a study that compared the effects of conventional estrogen replacement therapy with black cohosh. That study looked at 60 women, younger than 40 years old, who had had complete hysterectomies and were experiencing abrupt menopause. In all groups, treatment with black cohosh compared favorably with conventional treatment."
"Native Americans used the roots and rhizomes of this member of the buttercup family to treat kidney ailments, malaria, rheumatism, and sore throats. Early American settlers turned to it for bronchitis, dropsy, fever, hysteria and nervous disorders, lumbago, rattlesnake bites, and yellow fever. It's also reportedly well known for easing PMS and menstrual irregularities."
This estrogenic activity, notes Dr. Duke, can contribute to a 'mastogenic' effect; the natural enlargement of the breasts. Black Cohosh has also been used to induce labour and should not be used during pregnancy.
A dozen studies or more conducted throughout the 1980s and 1990s confirm that the long-standing use of black cohosh for menopausal symptoms has scientific validity. For example, in a German study involving 629 women, black cohosh improved physical and psychological menopausal symptoms in more than 80% of the participants within four weeks. In a second study, 60 menopausal women were given black cohosh extract, conjugated estrogens, or diazepam (a leading anti-anxiety medication) for three months.
Those who received black cohosh reported feeling significantly less depressed and anxious than those who received either estrogens or diazepam. In another study, 80 menopausal women were treated for 12 weeks with black cohosh extract, conjugated estrogens, or placebo. Black cohosh improved anxiety, menopause and vaginal symptoms. In addition, the number of hot flashes dropped from 5 to less than 1 average daily occurrences in the black cohosh group compared to those taking estrogen in whom hot flashes dropped from 5 to 3.5 daily occurrences.
Given these examples, and results of other studies, some experts have concluded that black cohosh may be a safe and effective alternative to estrogen replacement therapy (ERT) for women who cannot or will not take ERT for menopause.
Preliminary studies also suggest that black cohosh may help reduce inflammation associated osteoarthritis and rheumatoid arthritis. In a review of scientific studies, researchers concluded that a combination of black cohosh, willow bark (Salix spp.), sarsaparilla (Smilax spp.), guaiacum (Guaiacum officinale) resin, and poplar bark (Populus tremuloides) may help relieve symptoms of osteoarthritis.
For more information on Black Cohosh visit drugdigest.org.
Black Cohosh Benefits
http://www.herbwisdom.com/herb-black-cohosh.html#notes-side-effects
Black Cohosh has been used by Native Americans for more than two hundred years, after they discovered the root of the plant helped relieve menstrual cramps and symptoms of menopause. These days it is still used for menopausal symptoms such as hot flashes/flushes, irritability, mood swings and sleep disturbances. It is also used for PMS, menstrual irregularities, uterine spasms and has been indicated for reducing inflammation associated with osteoarthritis, rheumatoid arthritis and neuralgia.
Herbal researcher Dr. James Duke has this to say about Black Cohosh; "Black cohosh really should be better known in this country, especially with our aging population and the millions of women who are now facing menopause. Recognized for its mild sedative and anti-inflammatory activity, black cohosh can help with hot flashes and other symptoms associated with that dramatic change of life called menopause. It's also reported to have some estrogenic activity. Herbalist Steven Foster refers to a study that compared the effects of conventional estrogen replacement therapy with black cohosh. That study looked at 60 women, younger than 40 years old, who had had complete hysterectomies and were experiencing abrupt menopause. In all groups, treatment with black cohosh compared favorably with conventional treatment."
"Native Americans used the roots and rhizomes of this member of the buttercup family to treat kidney ailments, malaria, rheumatism, and sore throats. Early American settlers turned to it for bronchitis, dropsy, fever, hysteria and nervous disorders, lumbago, rattlesnake bites, and yellow fever. It's also reportedly well known for easing PMS and menstrual irregularities."
This estrogenic activity, notes Dr. Duke, can contribute to a 'mastogenic' effect; the natural enlargement of the breasts. Black Cohosh has also been used to induce labour and should not be used during pregnancy.
A dozen studies or more conducted throughout the 1980s and 1990s confirm that the long-standing use of black cohosh for menopausal symptoms has scientific validity. For example, in a German study involving 629 women, black cohosh improved physical and psychological menopausal symptoms in more than 80% of the participants within four weeks. In a second study, 60 menopausal women were given black cohosh extract, conjugated estrogens, or diazepam (a leading anti-anxiety medication) for three months.
Those who received black cohosh reported feeling significantly less depressed and anxious than those who received either estrogens or diazepam. In another study, 80 menopausal women were treated for 12 weeks with black cohosh extract, conjugated estrogens, or placebo. Black cohosh improved anxiety, menopause and vaginal symptoms. In addition, the number of hot flashes dropped from 5 to less than 1 average daily occurrences in the black cohosh group compared to those taking estrogen in whom hot flashes dropped from 5 to 3.5 daily occurrences.
Given these examples, and results of other studies, some experts have concluded that black cohosh may be a safe and effective alternative to estrogen replacement therapy (ERT) for women who cannot or will not take ERT for menopause.
Preliminary studies also suggest that black cohosh may help reduce inflammation associated osteoarthritis and rheumatoid arthritis. In a review of scientific studies, researchers concluded that a combination of black cohosh, willow bark (Salix spp.), sarsaparilla (Smilax spp.), guaiacum (Guaiacum officinale) resin, and poplar bark (Populus tremuloides) may help relieve symptoms of osteoarthritis.
For more information on Black Cohosh visit drugdigest.org.
JIM GREEN STUNNING RESEARCH ON ANTI AGING
http://www.greenray4ever.com/longevity2.html#ASTRAGALUSPREPARATIONSFROMVENDORS
http://www.greenray4ever.com/longevity2.html#ASTRAGALUSPREPARATIONSFROMVENDORS
Mini satellite DNA 5'-TTAGGG-3' repeats and searching for a heavenly connection to the starry dynamo in the machinery of night...
Anti-Aging Medicine: Life Extension in Review || 1 | 2 | 3 | 4 || Index Change Log
Longevity 1 | 2 | 3 | 4 | Bibliography | Labs 1 | 2 | 3 | Foots | Refs | Sup Notes 1 | 2 | 3a | 3b1 | 3b2 | 3b4 | 3b5 |
3b6 | 4 | 5 | Vendors | Change Log | Index Change Log | Age Transformation | Special: Restoring Senescent Cells
Topics | Am | An | B | Ca | Cb | D | E | F | G | Hs | Ht | I | J | K | L | Mc | Md | N | O | Pq | Pr | Q | R | Sl | Sm |
Te | Tf | U | V | W | X | Y | Z | Topics | More Topics | Health Topics || Home | Dental
JenAge/Blogs | Anti-Aging Firewalls | Fighting Aging | Biology of Aging/Blogs | MethusFoundationBlog | Biosingularity | @aging Blog | LifeExtension Blog | William Faloon | Dr. Jerry Shay | Dr. Woodring E. Wright
Videos : Immortality & Anti-Aging Medicine & Telomeres & Telomerase & Longevity & Medicine & Stem Cells & DNA Science & Gerontology & DNA Damage || Dr. William Andrews | Dr. Titia de Lange
& DNA Repair & Telomeres & Telomerase & E. Blackburn & Cellular Senescence & Replicative Senescence & M.Fossel & Nutraceuticals (HPlan) & Neuro & Aubrey de Grey: SENS Therapies || SENS | Henry Stewart Talks
& Cells & Skin & Detox & Heart & Cancer & Stroke & Neck Rejuvenation [Exercises] & Facial Exercises || Senior Journal || Solaray | Herbal Remedies | QC | Ray | AC-11 | Barron/End of Old Age & Dr. Carol Greider
Mechanisms of Aging | INNOVITA | SENS | Fight Aging | Methusalah Foundation | JenAge || Essential Evidence Plus | MIT | Molecular Biology of Aging Refs 2 | Hayflick Limit Papers | Longevity Genes
PubChem | PubMed | Labome |[ Wikipedia | Wikigenes | iHOP | SA Biosciences ]| Geron/Telomerase A || Cycloastragenol+: TA Sciences | Iron-Dragon | King Tiger | Genescient | Dr. Al Sears
AGE inhibitors | Alzheimers | Anti-Inflammatory | Cardiology | Antioxidants || Anticancer || Telomerase Inhibitors || Telomerase Activators | Sierra Sciences | IsAGenix | Product B | Terraternal | RevGenetics
Search: Journals | Basic Calc | Sci Calc | PubMed | NIH | MedLine | WebMD | HealthLine | Access Medicine | Wiki/Medicine | Medical Diagnosis | Truth In Aging || Clinical Trials | BioMedSearch | Discovery Medicine/Telomere
Google |[ Patents | Patent Lens ][ Books | LibCong | Amazon | Powells ][ Google Scholar | Wikipedia ][MedLib | LibWeb] | Merriam-Webster | Cope | HUGO | U.S. National Library of Medicine | Discovery Medicine
| LEF| Aging Cell| Rejuvenation Research| IAAS| A4M| ARC| ImmInst| SWL | Groups/LEF | Guide | MF | Immortal Humans
(6) The insulin utilization theory [Index/Insulin, Links/Insulin and Aging, Images, Papers, Patents, Books; Books/insulin_resistance, Amazon, LifeExtension/insulin_and_aging,
LifeExtension/insulin_utilization; Index/Diabetes].
Chromium picolinate can be used to metabolize extra insulin.
Treatment with chromium picolinate [Images] has been shown to increase the life span of mice 15%. There are some reports that chromium picolinate may be harmful to DNA [Links], and is primarily useful in losing weight. Perhaps some other source of the chromium ion should be used to impact insulin metabolism and help with the weight loss factor related to the caloric restriction theory (3). See also insulin and insulin resistance, Dr. Lam's Insulin and Aging, and Life Extension Magazine on metabolic syndrome and insulin resistance. It is desirable to avoid or deemphasize sugary foods and consume foods with a low glycemic index (*, Links) that cause glucose to rise more slowly to avoid obesity, metabolic syndrome and insulin resistance problems.
Glucose has a glycemic index of 100. Metformin makes cells less insulin resistant, and testosterone from bodybuilding, injections, or stick-on patchesprevents and treats insulin resistance in men. "Hyperinsulinemia (excess insulin in the blood) has been implicated as a major risk factor" in numerous age-related diseases, such as Alzheimer's disease, heart attack, and stroke. (See Fantastic Voyageby Ray Kurzweil and Terry Grossman [Clinic, Ray & Terry's]). Alpha lipoic acid has been shown to improve insulin-stimulated glucose disposal at 100-300 mg twice a day, and drugs used to treat metabolic syndrome include chromium 200 mcg x 2 or 3/day, alpha lipoic acid, EPA/DHA (fish oil) 1000 mg/day, co-enzyme Q 60-100mg x 2, Carnosine 500 mg x 2, magnesium 200-400 mg/day, CLA 500-1,500 mg x 2/day, 500-1,500 mg x 2, L-carnitine 600 mg x 2 or 3 per day, vitamin E400-800 IU/day, vitamin C 2000 mg/day, biotin, arginine 3 grams x 3, glutamine 500-1000 mg, DHEA 25 mg x 2, and N-acetyl-cysteine (NAC) 500 mg x 2/day. Otherwise, the glycemic index of food may be effectively lowered by dietary fiber or by Precose, and Glyset prescription medicines, which slow down absorption of carbohydrates from the digestive tract and by methods from caloric restriction.
The more sugar insulin transports into cells [Books], the more vulnerable the cell to glycation reactions [Papers, Patents, Books] producing carbonylated proteins [Images, Papers, Patents, Books; Patent] that may clog up proteasomes (14). Glycation reactions with DNA [Papers, Patents, Books] may also take place. This is probably the reason that genes like daf-16 [Links, Images, Papers, Patents, Books] in C. Elegans involved in insulin management are deemed "longevity genes" [Links, Images, Papers, Patents, Books; 0.2].
Note that for bodybuilding purposes, it is desirable to elevate insulin levels after an evening workout before bedtime to sweep nutrients into cells and restore muscle glycogen with insulin-boosting drugs such as Gymnema Sylvestre or Fenugreek extract, using a two-week on/off cycle to prevent breast enhancement. Elevating insulin makes one tired as blood glucose goes down, and eventually hungry, too.
(7) The advanced Hayflick cell division limit theory based on telomeres [Refs1b.7, Refs2.7, Fortune/Youth Pills, Books, Amazon, LifeExtension/telomere_therapy, Mechanisms of Aging/telomeres, Innovita, End Replication Problem, History of Telomeres and Telomerase, History of Telomerase Activators, Hayflick, His Limit, and Cellular Aging by Jerry Shay and Woodring E.Wright, telomeres & mortality, Ben Best/Telomeres and Aging; Mouse, the Unbeliever: SenescenceInfo/Telomeres & Telomerase, and Geraldine Aubert and Peter M. Lansdorp, Telomeres and Aging, Physiol. Rev.88: 557-579, 2008.] - Revolutionary advanced methods based on telomerase technology or alternate technology for allowing mitotic cell division to continue indefinitely [Geron] following gene therapy or alternative treatment to re-extend telomeres with telomerase activators [Index, List, 81s]. In vertebrates, telomeres at chromosome tip-ends [Books] consist of tandem arrays of minisatellite [5'-GGTTAG-3']n repeats from 4,000 to 15,000 bp long. These are often described as T2AG3repeats in the literature (5'-TTAGGG-3') (E. Blackburn, 2001). I have specified the sequence as [5'-GGTTAG-3']n (or G2T2AG), following the template region of the hTR RNA molecule (W Klapper et al, 2001, in Mark P. Mattson, 2001). "The template region of TERC is 3'-CAAUCCCAAUC-5'. This way, telomerase can bind the first few nucleotides of the template to the last telomere sequence on the chromosome, add a new telomere repeat (5'-GGTTAG-3') sequence, let go, realign the new 3'-end of telomere to the template, and repeat the process. " - Wikipedia/Telomerase.
TELOMERASE TERC Template RNA (hTR template RNA) 3'-CAAUCCCAAUC-5' ...GGTTAGGGTTAGGGTTAGGGTTAG-3' New Telomere Hex Repeat DNA
The first 5 nucleotides of the RNA TERC (hTR) template bind to the last 5 GTTAG nucleotides (shown in red) of the last hex repeat. After formation of the new DNA hex repeat GGTTAG the TERC (hTR) template steps right 6 nucleotides to align the next new hex repeat. "Normal human cells stably expressing transfected telomerase can maintain the length of their telomeres, and exceed their maximum lifespan by more than fivefold." Furthermore, normal cells immortalized with telomerase do not become cancerous [article], although 85% of known cancer cells express telomerase to enable them to keep dividing. In fact, it is believed that cellular telomeric crisis [Index/Telomere Fusion, Links, Images, Papers, Patents, Books, Amazon] due to shortening of cellular telomeres causes carcinomas [in breast cancer, Books]. Cytological observations of broken chromosome ends [Links, Images, Papers, Patents, Books, Amazon] by McClintock in maize in the 1940s [Books] led to the suggestion that telomeres functioned to prevent chromosomal end-point fusions, which have been recently shown to sometimes lead to cancer. Sperm cells and oocytes (germ cells) manifest high telomerase levels without showing cancer problems. When telomerase is applied exogenously to mitotic human cell cultures, they become immortal. Telomerase therapy even works to improve life span in long-telomere animals such as mice [Ref, 2012].
The Structure and Location of hTERT and hTR telomerase components
The hTERT component
I note that the hTERT protein catalytic component of telomerase is generated by the hTERT gene [Index, Links, Images, Video, Papers, Patents, Books, Amazon], consisting of 16 exons and 15 introns spanning ~37 kb of genomic sequence (Wick et al., 1999) near the distal end of chromosome 5p at 5p15.33, probably the most distal gene on the chromosome, which contains 609 genes. The half-life of the associated molecule (hTERT mRNA transcript half-life (1-3 hours), hTERT protein half-life (4 weeks), half-life of the active telomerase complex (24 hours)) is up to 4 weeks. (See Xiaoming Yi, Jerry W. Shay, and Woodring E. Wright, Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells, Nucleic Acids Res, 2001 December 1; 29(23): 4818–4825). The hTERT protein, the hTERT catalytic component of telomerase, includes 1132 amino acids with a total molecular mass of 127 kDa. The information for the transcribed part of hTERT mRNA is contained in 1132 x 3 nucleotides + 3 stop nt + 3 start nt = 3402 nt, and together with the 5' cap, the 5' UTR, the 3' UTR and the poly(A) tail of mRNA structure, hTERT mRNA is contained in 4015 nt. One microgram of 20 nt of single-stranded RNA yields 9.17 x 1013 molecules, so 1 microgram of 4015 nt of hTERT mRNA yields (20/4015) x 9.17 x 1013 = 457 x 109 hTERT mRNA molecules. For purposes of viral or plasmid transfection, it may be useful to prepare condensed hTERT cDNA with various promoters.
Adding the 16 exon lengths of hTERT together, I find 3396 nt length, 3.396 Kb. The CMV promoter length is 508 nt, so that hTERT cDNA with a CMV promoter and without introns should come to 3904 nt or 3.904 Kb. Using the canonical 181-bp hTERT core promoter 19 bp upstream of the first nucleotide in the cDNA sequence instead would yield hTERT cDNA + core promoter = 3396+181+19 nt = 3596 nt = 3.596 Kb. The hTERT protein structure is highly similar to retrotransposons and includes the motifs 1, 2, A, B', C, D, and Echaracteristic of the active sites of retrotransposons. In addition, telomerases contain the hTERT T-motif and show a larger distance between the A and B' motifs than is found in retrotransposons. Our problem of telomerase activation to repair shortened old telomeres and allow more cell divisions is primarily dependent on our ability to supply, use, or activate transcription factors that interact with the hTERT promoter to produce hTERT mRNA used to produce the catalytic component of telomerase. This process may be accelerated by HDAC inhibitors that act to expand chromatin by acetylating DNA, promoting transcription and counteracting gene silencing. Otherwise, telomerase activation is typically produced by phosphorylating cytoplasmic hTERT with kinases (such as AKT1 kinase) for import into the nucleus. Fast telomere extension using purified nucleoside-modified hTERT mRNA in liposomes was announced in 2013. This method extends telomeres in six days by an amount by which telomeres shorten over approximately 15 years of normal human aging.
The hTR component
The hTR sequence for the RNA polymerase II-transcribed RNA part of telomerase is located on chromosome 3 (1,436 genes) at 3q26.3, and the associated hTR mRNA exhibits a half-life of about 5 days. Check the length of the hTR mRNA transcript. All vertebrate hTR RNA uses the template yielding the hex nucleotide DNA extension sequence [5'-GGTTAG-3']n, includes up to 35 RNA sequences, and features 4 conserved structural domains: the CR/C5 region, the psuedo-knot, the H/ACA box (for localization of telomerase hTR RNA to the nucleolus), and the CR7 region (W Klapper, R Parwaresch and G Krupp, 2001). It seems likely that the H/ACA domain of hTR RNA binds to H/ACA proteins formed by snoRNAs such as dyskerin, which is found in the nucleolar telomerase complex.
In adult tissues, hTR is present and most highly expressed in the spermatocytes and Sertoli cells of the testis, moderate expression is observed in germinal center lymphoid follicles, and weak expression is present in regenerative cellular epithelia, but is not seen in the nervous system and mesenchymal derived tissues including connective tissue, bone, cartilage, and the circulatory system and lymphatic system cells. Treatment with mesenchymal stem cells is one method of handling their eventual failure due to low hTR expression in regenerative medicine.
See Nedime Serakinci, Stacey F Hoare, Moustapha Kassem, Stuart P Atkinson & W Nicol Keith (2006), Telomerase promoter reprogramming and interaction with general transcription factors in the human mesenchymal stem cell, Regenerative Medicine, Jan 2006, vol.1, No.1, pp 125-131 (Summary in Future Medicine). "It is shown that repression of hTERT expression in hMSCs (human Mesenchymal Stem Cells) is due to promoter-specific histone hypoacetylation coupled with low Pol II and TFIIB trafficking. This repression is overcome by treatment with Trichostatin A (TSA), an HDAC inhibitor, concomitant with increases in promoter-specific histone acetylation and increases in Pol II and TFIIB tracking. hTR expression is also increased in TSA-treated hMSCs, concomitant with changes in Pol II and TFIIB dynamics." It is probably true that hyperacetylation with sodium butyrate or sodium butyrate chicken feed would produce equivilant results.
Garlic improves the Hayflick limit of dermal fibroblasts, probably due to the HDAC inhibitor properties of diallyl disulfide and allyl mercaptan metabolites of allicin from garlic, which expand chromatin for better transcription of hTR and hTERT mRNA. HDAC inhibitor properties have recently been discovered for L-carnitine, so that this common nutraceutical (obtainable from methionine from fish in the presence of lysine and vitamin C) might also be used to overcome poor expression of hTR and hTERT in mesenchymal-derived tissues via hyperacetylation of chromatin. See hTR and the hTR Promoter. Several "putative" binding sites for the glucocorticoid, progesterone [hTERT_promoter], and androgen steroid hormones have been found in the 5' flanking region of the hTR gene and may also be present in the hTERT gene (C.J. Cairney and W.N. Keith, 2007, 2008).
Expression of hTR in adult tissues is predominantly limited to dividing cells, although certain differentiated postmitotic cells express hTR. [Yashima K, Wright WE, Shay JW, et al., 1998, Papers/hTR activation, Papers/small molecule hTR activators, J. Zhao, et al, 2003, Links/telomerase RNA gene activation, Links/hTR plasmids, Links/hTR transfection]. The hTR or hTERC promotor is silenced via methylation [Index, Links, Papers]. Sp1 (binding at GC boxes) and HIF-1 are positive regulators of hTR transcription (C.J. Cairney and W.N. Keith, 2007). Note HIF-1 can be upregulated by hypoxia from exercise, ginkgo biloba, or by fenugreek seed, while Sp1 is upregulated by sodium butyrate [Images] and down-regulated by insulin deprivation. Perhaps these can be applied to solve the special problems involving mesenchymal-derived tissues. Note, however, that sodium butyrate induces growth arrest (in cancer cells) by inhibiting DNA synthesis, arresting actively proliferating cells in G1 to induce differentiation. This suggests that perhaps it should not be used continuously. Sodium butyrate supplements are sometimes shown with a rooster-waggle neck picture, probably to suggest that they may be good for neck wattles composed of old connective tissue derived from mesenchymal cells by boosting the expression of hTR there via elevated Sp1. Increased Sp1 binding to promoter regions is seen in cells that overexpress the endogenous antioxidants Cu/Zn-SOD (SOD1) and/or catalase [Index], and curcumin has been observed to increase Sp1 binding. Recently it has become clear that hTERT and hTR are better expressed in mesenchymal-derived tissues when HDAC inhibitors such as sodium butyrate or L-carnitine are used to expand chromatin for transcription.
Telomerase Fractionation
Fractionation of the telomerase enzyme from nuclear extracts with a glycol gradient yields a size of about 1000 kDa for the native enzyme and about 550 kDa for affinity purified telomerase, the difference being due to loosely associated factors lost during purification (W.Klapper et al 2001). A single hTERT molecule accounts for just 127 kDa. In addition, we have the hTR RNA, dyskerin, the shelterin or telosome proteins, and associated mammalian telomere protein factors. Two molecules of dyskerin, two molecules of hTERT protein catalytic component of telomerase, and 2 molecules of telomerase RNA are found in telomerase reverse transcriptase, according to recent research. This may be two molecules of telomerase bound at the RNA template, however. See Scott B. Cohen, Mark E. Graham, George O. Lovrecz, Nicolai Bache, Phillip J. Robinson, Roger R. Reddel, (2007), Protein Composition of Catalytically Active Human Telomerase from Immortal Cells, Science, 30 March 2007: Vol. 315. no. 5820, pp. 1850 - 1853. "Dyskerin is a component of a small nucleolar ribonucleoprotein(snoRNP) that is highly conserved throughout evolution.... findings indicate that dyskerin plays a role in telomeremaintenance by stabilizing TERC." - - H.-Y. Du et al. Telomerase Mutations and Premature Aging in Humans.
Molecular Densities of Telomerase
The abundance of telomerase molecules is low in cells, featuring for instance 3000-5000 copies of hTR and 30 copies of hTERT protein per cell in in vitro cultures of growing tumor cells that are the richest source of telomerase. (W. Klepper, et al. 2001). Of course, we may also inquire concerning the abundances of telomerase, hTR RNA, hTERT protein, and dyskerin molecules in sperm cells, dermal fibroblasts, hemopoietic stem cells, immortalized cells, and other types of cells under varying conditions at as a function of age.
Telomere Lengths
The lengths of different chromosomes correlate directly with telomere length [Images], and the telomeres are shorter the closer they are to the centromere [Images]; p-arm telomeres are shorter than q-arm telomeres [Images]. The lengths of telomeres may vary between alleles at the same telomere in a diploid cell. The shortest telomere in human cells is at 17p[Links, Images, Papers, Books, Wikipedia/Chromosome 17, Ornl, Genes & Disease]. The hTERT catalytic component of telomerase available is the primary limiting factor in telomerase expression, and transcriptional regulation of hTERT gene expression rather than of hTR for the RNA component of telomerase is central in replicative senescence and immortalization. Replicative senescence is pro-inflammatory and pro-carcinogenic, although it limits uncontrolled tumor growth. Inhibiting telomerase causes cancer cells to self-destruct via apoptosis unless telomerase control is via the ALT mechanism, so that telomerase inhibitors are often useful in squelching cancers. Mammalian telomeres of maximum reported length 30,000 base pairs shorten by 50-200 base pairs with each S phase of the cell cycle, starting with typically 15,000 to 20,000 base pairs in the germ line with typically 7,000-10,000 base pairs in human adults, and counting down to 4,000-7000 base pairs in senescent cells at the M1 senescent cell checkpoint preceding a conditionally triggered countdown to the M2 crisis state before apoptosis, end-point fusions, and other phenomena associated with cell death or cancer set in.
Human germline cell telomeres are maintained at about 15 kbp. Telomeric t-loops [Index] thousands of base pairs long are uncapped to reach the M1 senescence checkpoint, when the length of telomere has shrunk so that the loop tries to close at a spot where non-canonical subtelomeric repeats are encountered differing from the tandem telomeric hexanucleotide repeats [5'-GGTTAG-3']n, so that the TRF1 t-loop formation protein [Index] closing the t-loop cannot find a proper binding site. In experiments, fibroblasts [Images] with telomeres shortening up to 200 base pairs per cell division were found to loose only 5-20 bp per cell division when the cell was equipped with the most effective antioxidant defense. (This might motivate using lipid-soluble antioxidants along with water-soluble oxidants like vitamin C, or antioxidants soluble in lipids and water like alpha lipoic acid, the recommended alternative.) Cells of the healthy colon mucosa typically loose 44 base pairs per cell division. On the other hand, patients 77.5 years old were shown to have white blood cells that lost 71 telomeric base pairs per year [Nazmul Huda, et.al, 2007], perhaps showing some old-age acceleration in telomere erosion. The same paper showed that telomere lengths nearly matched between elderly twins. In one 2003 study, patients over 60 years old with shorter telomeres in blood DNA had poorer survival than patients with longer telomeres, attributable in part to a 3·18-fold higher mortality rate from heart disease and an 8·54-fold higher mortality rate from infectious disease. (Richard M. Cawthon, Ken R. Smith, Elizabeth O'Brien, Anna Sivatchenko, Richard A. Kerber, 2003). Other studies have pictured more precisely how mortality varies with telomere length in blood leukocytes, and shown how cancer incidence and mortalityvary as a function of telomere length.
Telomerase and Aging in Non-Mitotic Cells and Mitochondria [Links, Images, Papers, Patents, Books].
Note that replicative senescence does not apply in quite the same way to non-mitotic cells such as muscle cells [Links], muscle satellite cells [Links], cardiac myocytes [Links], and most nerve cells, which appear to age via other mechanismsinvolving the cell membrane, cell membrane permeability changes from hydroxyl radical reactions, membrane damage from ROS, accelerated ROS from AGEs, and internal waste accumulation. (Sometimes such cells are renewed by stem cells from bone marrow, as in the the case brain microglial cells [77s], so that the telomeric state of mitotic stem cells may be behind the continued health of non-mitotic tissue.) However, Geron claims that in vitro cultures of non-mitotic nerve and muscle cells as well as mitotic cells are functionally benefited by telomerase activation, listing 20 cellular types [81s]. It was later discovered that the hTERT protein defends mitochondrial DNA and improves DNA repair in nuclear DNA. "In many cases, introduction of active telomerase also increases the capacity of cells to withstand stress due to high or low levels of oxygen, toxic molecules, or abnormal growth conditions." Experiments in 2007 have succeeded in immortalizing muscle cells in in vitro culture using a combination of hTERT and cyclin-dependent kinase 4.
See cyclin-dependent kinase 4 [Papers, Patents, Books], which uses ATP to phosphorylate proteins and is subject to inhibition by p16INK4A and other INK4 family members [Ref]. When grown in in vitro cultures, human (73-78 year lifespan) embryonic cells divide about 50 times before reaching their telomere-imposed cell division limit, mouse (3 year life span) cells divide 15 times, and the cells of Galapagos tortoises (175 year life span) divide 90 times. Cells from human adults, on the other hand, were observed to typically divide 20 times, or 50 times from the embryonic stage of development. Telomerase activation by small molecule telomerase activators [81s/TA, List, Index] such as astragalus root extract, black cohosh, fenugreek extract, testosterone, colostrum growth factors, Nitric Oxide (NO) from arginine, HGH from alpha-GPC with exercise, IGF-1, or CGK1026, however, can confer an immortal phenotype on cells by bypassing the usual cellular senescence pathway, and can restore a youthful phenotype to aging cells with short telomeres.
Carnosine and Antioxidant Telomere Defense
"In 1999, Australian researchers confirmed that carnosine increases the longevity of human fibroblast cells in the laboratory. Carnosine extended the Hayflick limit (the maximum number of times a cell can divide), from a "normal" 50 by up to an additional 10 times!" [69] See Life Extension on Carnosine and Cellular Senescence and The Barron Report. Note that von Zglinicki et al. showed that cells grown in a high oxygen atmosphere experience increased telomere loss and increased single-stranded telomere breaks, then prematurely senesce. So any drug protecting telomeres against oxidative stress should help, including the "super antioxidant" carnosine [69]. Reducing oxygen in a cell culturing system sometimes yielded cells capable of 3x as many cell divisions [Zs. Nagy, The Membrane Hypothesis of Aging, p.8.].
Aging without Telomere Shortening
Also, note that the fibroblasts of the mouselike dunnart double 170 times, but the animal only lives 3 years, no doubt because the other mechanisms of cellular senescence kill it before replicative senescence does [Zs. Nagy, p.8]. Similarly, it is surprising that the mouse has telomeres > 25,000 base pairs long; typically dying not of replicative senescence, but of mitochondrial failure, which is typical of animals very subject to predation. The relatively safe mouse-like bat lives 40 years, enjoying telomere-controlled longevity. Mice with the hTR RNA component of telomerase knocked out show no old-age effects due to telomere shortening until the 6th generation. Coincidentally, the mouse is more prone to cancer than humans; mouse telomeres are so long that they rarely catch runaway cell division. Telomeres sometimes stop the runaway cell division associated with cancer unless the cancer causes the gene to express telomerase. Syrian hamster embryo cellsexpress telomerase to keep their cellular telomeres long for 20-30 population doublings until they senesce by another mechanism.
"Normal somatic cells are generally telomerase negative, except for bone marrow stem cells." Note that germ cells express the most telomerase, maintaining their telomere length constant, while stem cells senesce more slowly than normal somatic cells because they intermittantly express telomerase [M.Fossel, Cells, Aging, and Human Disease, p.54].
Neygeront and Kinetin
Another Hayflick limit extender is the RNA and B-vitamin complex Neygeront [79], which may boost the number of possible cell divisions by 20%. Kinetin (N6-furfuryladenine), in carefully controlled doses, may be another Hayflick Limit extender. Kinetin, a cytokinin pioneered in anti-aging research by Dr. Suresh Rattan, (also a student of zeatin) is presently being used on skin cells. Mind you, we are sometimes up against the problem: "The senescent phenotype was not prevented... although telomerase activity was induced...", the solution to which we must seek in other sections of this and similar essays. For instance, activating telomerase to lengthen telomeres saves fibroblasts, but not pancreatic islet cells, from senescence.
P16INK4A and the Reversibility of Senescence
P16INK4A can be too high for replicative senescence to be reversed by telomerase activators [Index], but can sometimes be kept within bounds by nerve growth factor, which produces Id-1 helix-loop-helix transcription factor limiting P16INK4A. Note that vitamin A or retinol from carrots, sources of the telomerase inhibitor retinoic acid, reduce P16INK4A expression. However, retinoic acid represses hTERT transcription, so that vitamin A, carrots, retinol, and retinoic acid are to be taken during telomerase activation OFF phases of treatment. P16INK4A is a ubiquitinated protein that is devoured (the rate of ubiquitination depending on P16INK4A density), so that reducing its expression really reduces P16INK4A levels in the cell. See (Ronen Ben-Saadon, Ifat Fajerman, Tamar Ziv, et al, 2004). Resveratrol's heightened SIRT1 expression downregulates P16INK4A expression, retarding the onset of irreversible replicative senescence. Also note that exercise can bring P16INK4A expression down by 2 binary orders of magnitude. High P16INK4A levels are often associated with aging, smoking, and physical inactivity.
Heat Shock Proteins Increase Telomerase Levels
Another method of increasing telomerase levels in the cell is via heat shock proteins, which mediate the assembly of telomerase (GB Morin, DO Toft, JW Shay, WE Wright, MA White, et al., (1999)). Note that HSP90 levels may be elevated for transport of nuclear transcription factors into the nucleus via the action of alpha lipoic acid or via interleukin 6 produced by exercise. HSP90 also guides protein folding as a chaparone.
Telomerase Activation by Nitric Oxide in Endothelial Progenitor Cells
A technique described by Vasa, et. al., used Nitric Oxide to activate telomerase and delay senescence in endothelial cells. See Nitric Oxide Activates Telomerase and Delays Endothelial Cell Senescence and Hayashi, et.al, 2006. Since NO reacts with the superoxide anion to form peroxynitrite, which produces hydroxyl radicals, it is probably a good idea to use the peroxynitrite inhibitor gamma-tocopherol when using NO to promote telomerase activation. Bodybuilding exercisespromote Nitric Oxide synthase production, and in the presence of arginine this leads to Nitric Oxide generation [Books/Nitric Oxide] suitable for telomerase activation and promotion of mitochondrial biogenesis [Books]. Nitric Oxidegeneration is also promoted by broccoli sprouts, genistein, and resveratrol [Links, Books]. Finally, the release of NO in endothelial tissues is promoted by acetylcholine, which may be boosted with ashwagandha, huperzine A, or acetyl L-carnitine. Nitric Oxide release for purposes of telomerase activation might be best promoted in the vascular endothelium by bodybuilding workouts in presence of arginine and ashwagandha, huperzine A, or acetyl L-carnitine as supplements, to which we might add broccoli sprouts, genistein, or resveratrol with gamma tocopherol to neutralize peroxynitrite.
Small Molecule Telomerase Activators
[Index, List, Wikipedia/Telomerase, Links/Histone Deacetylase Inhibitors and Small Molecule Telomerase Activators, Papers, Patents, Books; Index/HDAC inhibitors, Books/telomere homeostasis, Books/telomerase activators, Books/Small Molecule Telomerase Activators, Patents; Endogenous Telomerase Activators; Exercise-Induced Telomerase Activators; History of Telomerase Activators].
Geron Telomerase Activators
Geron [SEC filings, Investor Guide] and a subsidiary TA Therapeutics have announced a small molecule telomerase-activator TAT0002 (TAT2, cycloastragenol), orally bioavailable, presently used in AIDS therapy that may become useful in life extension work, say on T-lymphocytes and on on the skin [article], now to involve telomerase activation therapies[Index] or telomerase transcription-activation methods. Geron has also determined that its telomerase activators [List, Index] promote wound healing. [Geron/telomerase activators: Table]. Concentrations of astragalosides at 1 μg/ml are typical for topical applications or in testing for telomerase activation effectiveness using a telomerase TRAP assay [Books]. Telomerase activity has a half-life of about 24 hours. One of Geron's formulas [See also A' alternate-source version Compositions and Methods for Increasing Telomerase Activity, A'', Hong Kong Univ. formulas and Patent Lens] for a small-molecule telomerase activator is Astragaloside IV (molecular diagram, vendors RevGenetics, Terraternal), which the firm claims is associated with an optimal dosage of 50 mg to 100 mg per day (p.39-40). This looks like an overdose by a factor of 10 or 20, but that may be due to reduced bioavailability of astragaloside IV by itself.
Astragaloside IV is more bioavailable when taken in astragalus root extract, which is typically used to boost the immune system. The bioavailability of astragaloside IV[Article, Papers, vendors RevGenetics, Terraternal], can also be increased by chitosan [Index, Wiki/chitosan, Links/chitosan] or sodium deoxycholate [Index, Links, Books]. The small-molecule telomerase activator TA-65, a product of TA Sciences, primarily composed of cycloastragenol [Index, 81s/6b, Links, Images, Papers, Books] has also been announced by Geronalong with several other formulas based on astragalus root extract molecules [Geron European Patent and Hong Kong University European Patent]. TA-65 was initially prescribed at 5 mg/day, then finally at 25 mg/day. The 5 mg dose was recommended by Geron for cycloastragenol in their European patent (p.39-40). A 1/08/2011 email source quotes a chemical analysis showing that TA-65 is: 5.47 mg cycloastragenol + 0.27 mg astragaloside IV + < 0.01 mg astragenol per serving. (See page 65 for the molecule, and note that "cycloastragenol is the common genuine aglycone of the astragalosides", the smallest of the astragaloside-related telomerase activator molecules, resembling astragaloside IV with its two lower ball-like carbon rings off, as does likewise small astragenol (List). "One-nut" versions of astragaloside IV are described on page 66, cycloastragenol 6-β-D-glycopyranoside and cycloastragenol 3-β-D-xylopyranoside.) TA Sciences at first claimed that TA-65was "one single, potent molecule" from astragalus extract, thus probably cycloastragenol, and not astragaloside IV, which required 50 to 100 mg per day doses to be effective without excipients to improve bioavailability. TA-65 might have been astragenol, the dosage of which was unspecified in the Geron European patent, until chemical analysis emanating from RevGenetics determined that it was primarily cycloastragenol. Geron singles out cycloastragenol as having very low toxicity and excellent bioavailability on page 40 of Compositions and Methods for Increasing Telomerase Activity (or see A' alternate-source version Compositions and Methods for Increasing Telomerase Activity, or A''), and emphasizes a procedure for converting astragaloside IV chemically to cycloastragenol. TA-65 is applied by TA Sciences in staggered fashion 3 months on, three months off, and again three months on during a period of one year in the Patton Protocol.
It seems that after 3 months using TA-41 astragalus extract to test the Patton Protocol, TA Sciences saw 230 base pairs of telomere growth in their early studies [81s/6d]. By May 2012, it seems astragalus extract was more effective in producing increases in average telomere length than TA-65, cycloastragenol, or astragaloside IV, according to studies carried out by the VIDA Institute, Terraternal, and Calvin B. Harley, et al. (2011), in A Natural Product Telomerase Activator As Part of a Health Maintenance Program, Rejuvenation Research, 14(1), 2011. "Results of an informal, but scientifically conducted, several month trial with laboratory analysis of biomarkers, in fact, showed that a combination of whole astragalus root, and natural astragalus extract was an order of magnitude more effective than either cycloastragenol or astragaloside IV at increasing the telomere length of immune system (NK) cells in the blood.... Using Standardised Astragalus Root Extract (+ physical training, meditation, less work, gingko biloba, orlistat and LCHF ).... The median telomere length of my NK cells increased in length by a whopping 1,800 base pairs (in 3 months)." - VIDA Institute. The VIDA Institute recommends taking ginkgo biloba (which makes blood platelets less sticky) and gotu kola (Centella Asiatica) with astragalus root extractto improve its access to the fine structure of the circulatory system, so that it can reach more cells.
About 50-200 telomere base pairs are lost per cell division, a number than can be reduced to from 20 bp to 5 bp by taking antioxidants. Note that humans typically have about 50 cell divisions available at the embryonic stage (except for stem cells), so that such a program of treatment might go on for years. Geron's preferred embodiments in 2005 for a small molecule telomerase activator are based on astragaloside IV, cycloastragenol, astragenol, and astragaloside IV 16-one, although they have named several other effective compounds also obtained from astragalus root extract, including cycloastragenol 6-β-D-glucopyranoside and cycloastragenol 3-β-D-xylopyranoside. Relatively highly bioavailable cycloastragenol may be obtained from astragaloside IV [RevGenetics] or by other methods from astragalus membranaceus. By the Spring of 2011, astragalosides seemed rather less effective than described above, working best as telomerase activators on NK cells, explaining why astragalus is good at deflecting viral infections. Approaches based on several telomerase activators in parallel, plus exercise with HGH secretagogues and support for endogenous telomerase activators, now seem more promising than total reliance on astragalosides or TA-65, based on studies of TA-65 performance by Calvin B. Harley and other leading investigators on data gathered since the spring of 2007. Geron has also described a "formula III" telomerase activator embodiment, ginsenoside RH1 [Index, List, Links, Papers, Patents, Links/ratios, Papers/ratios]. Red Korean Ginseng Extract may include some ginsenoside RH1 [molecule], but on the whole the extract turns out to be a telomerase inhibitor.
Astragalus Preparations from Vendors
In 2007 I thought the best and safest commonly available medicine for telomerase activation was GAIA Herbs Astragalus Root Extract featuring 30 drops/mg of astragalosides, given at 5 mg of astragalosides/day during 2-week activation time cells alternating with 2 week periods of telomerase inhibition with telomerase inhibitors like garlic, curcumin, resveratrol, quercetin, silymarin, melatonin, vitamin E, green tea EGCG, and fish oil's EPA. Since GAIA Herbs Astragalus Root Extractwas no longer available at 1 mg astragalosides per 30 drops, I at first (2008) substituted 1200 mg/day of Solaray Astragalus Root Extract to cover the 5 mg of astragalosides, which requires 6 x 200 mg capsules per day in a cyclic protocol featuring 15 days on, then 15 days off. By December 2009, I had to substitute 5 droppers of Herb Pharm Astragalus Root Extractplus 6 x 200 mg capsules of Solaray Astragalus Root Extract to approach the 5 mg of astragalosides per day requirement, adding 4 to 8 capsules of Natural Balance Chitosan to improve bioavailability. This was for the first two-week part of the cycle, which features telomerase activation. During the 2nd part of the month-long cycle, telomerase inhibitors are used instead, as described above. Although this exceeds the recommended dose according to Solaray, I believe from toxicologystudies [Index/Astragalus Extract] that it is safe enough. By 2011 I was able to obtain Astragaloside IV at 100 mg/day from vendors like RevGenetics and Super Smart, and cycloastragenol at up to 25 mg/day from several vendors.
By 2012 I dropped astragaloside IV and cycloastragenol, returning to 1/2 bottle of GAIA Herbs green label astragalus root extract per day (15 x 30 drops = about 15 mg astragalosides at the time) with 50 x 500 mg = 25 g) of NOW astragalus root powder in capsules, as it became clear that this was both more effective and more inexpensive. I put 3-5 droppers of the 1/2 bottle of GAIA Herbs green label astragalus extract into my scalp to stimulate hair follicle cycles. Then the cost is approximately 15 x ($10.50/2) = $78.75/month for the astragalus extract and ((50) x $5.91/100) x 15 = $44.32 for the astragalus root powder capsules, for a total of $123.08/month. Using just 5 mg of astragalosides/day from the extract, I might have cut the cost to ($78.75/3) + $21.00 = $47.45/month. Using exclusively NOW astragalus root powder capsules at 33 grams/day, the maximum dose of astragalus root powder used in Chinese medicine, I would have spent 15 x (66 caps) x ($5.91/100 500 mg caps) = 15 x $3.90/day = $58.51/month. To this one would typically add 3-5 droppers (30 drops/dropper = 1 mg astragalosides) per day of the GAIA Herbs green label astragalus extract for the scalp. This would cost 3-5 droppers x ($10.50/30 droppers per bottle) x 15 days, or $15.75/month for 3 droppers/day in 15 days of a month, up to $26.25/month for 5 droppers/day for 15 days of the monthly cycle. Then the cost would be less than $84.76/month for 33 grams/day of astragalus root plus a deluxe astragalus root extract scalp rub. See the complete current program for 2013, which features a number of telomerase activators and booster supplements for them.
Combinations of Telomerase Activators [List]
Combinations of other telomerase activators also became available, such as RevGenetics Astral Fruit NF and custom combinations including Fenugreek extract for diosgenin and 4-hydroxyisoleucine for an insulin boost to provide improved access by telomerase to telomere t-loops by phosphorylating tankyrase 1 for stripping TRF1 telomere loop closure proteinfrom telomeres in the presence of a NAD+ substrate provided by early morning niacinamide (nicotinamide). See Age Transformation for a logbook of this experiment. Telomerase inhibitors and telomerase activators are not to be taken at the same time. Reconstructing cellular telomeres using activation of telomerase closes chromosomal telomere t-loops in senescent cells, restoring the youthful phenotype and patterns of gene expression at a rejuvenation rate of up to 0.75 years per month (9 years/year, or 460 bp/year of telomere growth using the cyclic Patton Protocol). Another alternative is Herbal Remedies Astragalus Root Extract 1.25 mg astragalosides per 250 mg cap, via Nature's Way, ( Standardized 0.5% Astragalosides ), 60 VCapsules per bottle, incuding Astragalus, dried extract 250mg (root) 0.5% astragalosides, with Astragalus (root) 250mg. Four capsules yield 5 mg astragalosides plus 1 gram of astragalus membranaceus root, which improves bioavailability of astragalosides. Note that Ginsenoside RH2, a telomerase inhibitor, may also be present in ginseng extracts, in addition to telomerase activator ginsenoside RH1. This may be why Korean Red Ginseng is a telomerase inhibitor (31). Note that the ginsenosides [Index, Links, Books] are distinct from the astragalosides, being triterpene saponins [Index, Books] from ginseng [Index, Links, Books, in circulation].
One starting material from ginseng is panaxatriol. Geron also names astragalosides A, 1, 2, 7, and astraverrucins I and II, which can be obtained from Astragalus Verrucosis, as telomerase activators. TA-65 "is a naturally occurring molecule extracted from the well known Traditional Chinese herb Astragalus membranaceus [Index, LifeExtension, Solaray, GAIA Astragalus extract, 1 mg of astrogalosides per 30 drops]", also called Milk Vetch Root, or Huang-qi (Yellow Leader) [81s/6b]. However, there are indications that astragalus extract works best to increase telomere length in Natural Killer cells. Geron seems to have tested drugs and nutraceuticals known to boost the immune system in its search for small molecule telomerase activators [List], as both astragalagus root [Index] and ginseng root [Index] had been used in traditional medicine for the immune system. Both contain saponins [Index]. Perkins Coie has recently filed a patent application on behalf of Geron's Chief Scientific Officer Calvin B. Harley [Index, Forbes/Calvin B. Harley, Papers/Calvin B. Harley, Books, Portrait] for anti-aging skin creams using astragalosides. Liposome delivery systems [68] in connection with small molecule telomerase activator delivery look quite interesting.
Cut-Rate Telomere Therapy with Fenugreek, Black Cohosh, Exercise, HGH Secretagogues, Garlic, and Onions
Perhaps Fenugreek seed alone at 3 grams/day just before bedtime for 20 days of the month can preserve us at a specific age. Then telomere length might be held constant for just $5.00/month for 100 caps of 610 mg/cap Fenugreek seed from Wal-Mart, taken 5 caps/day for 3 grams/day for 20 days. Black cohosh for 25 days at 4 caps/day from a 100 caps for $5.00 bottle might also help stabilize telomere length at a reasonable price, or the two could be combined. Thus low-cost telomerase activators exist that may allow us to hang on as well-preserved cut-rate fellow travelers for 5 to 10 dollars/month, even when we can't afford $100/month for basic rejuvenation treatment with astragalus formulations. Exercise can be free and is a source of endogenous telomerase activators that can be boosted with nutraceuticals such as whey protein. HGH secretagogues in combination with exercise may be most useful, as virtually human cells have about the same density of HGH receptors in their cell membranes. Garlic [List] improves the Hayflick limit for dermal fibroblasts, and onions contain quercetin [List], which has some telomerase inducing power.
Telomolecular Nanotechnologies
Another firm, (now-defunct) Telomolecular Nanotechnologies [SEC data 11/7/06], also developed therapeutic products based on telomerase activation, and was developing products including rejuvenating skin creams based on telomerase activation. According to now-defunct Telomolecular Nanotechnologies (Corporate Video), youthful "telomerized cells" seem immune to free radical damage and glycation, due partly to the telomerase position effect described in subsequent section. "...Cells with sufficiently elongated telomeres energetically produce, in high levels, proteins like catalase, superoxide dismutase, glutathione, Ku, collagen, elastin and many other proteins important in tissue formation, cell repair, and antioxidation, that become scarce as telomeres shorten." (See Overexpression of Telomerase... by Armstrong, G. Saretzki, and Peters, et. al.)
DNA Rolling Nanocircle Encoding
Telomolecular Nanotechnologies proposed DNA nanocircles for telomere re-extension [Telomolecular Nanotechnolgies/nanocircles, Amazon, Links, Images, Papers, Patents, Books]. Produced by a DNA synthesizer, DNA rolling nanocircle encoding for telomere rejuvenation was invented by Dr. Eric Kool (article) of Stanford University. See Dr. Kool's patent, Telomere-Encoding Synthetic DNA Nanocircles, and Their Use for the Elongation of Telomere Repeats(More). However, DNA nanocircles for telomere extension are not ready for internal use at this time, but are only used for special applications and in the study of ALT mechanisms in cancer. "Telomolecular believes that nanocircles might work efficiently in living animals."
Direct hTERT Delivery to Cells
Another firm, Phoenix Biomolecular, has specified a cell penetrating peptide scheme to deliver hTERT directly to cells. It is subsequently necessary to phosphorylate hTERT then for transport into the cellular nucleus. Liposome preparations that enter the cell via endocytosis are promising both for large molecules and plasmids. Plasmids in which Green Fluorescent Protein is driven by a copy of the hTERT promoter on the plasmid may be used to guide cancer surgery, and finite-lifetime plasmids expressing C-myc may be used to enhance hTERT transcription levels.
hTERT mRNA Delivery to Cells
Fast telomere extension using HPLC-purified, nucleoside-modified hTERT mRNA to escape from the innate immune system, uses mRNA with amplified poly(A) tails that gradually shrink to a minimum length before the mRNA is consumed by an enzyme. The therapeutic hTERT mRNA may be transfected in liposomes. It is capable of lengthening telomeres in 6 days by the amount that telomeres shrink in 15 years of aging, so that it should be useful for stem cell transplants and rejuvenation.
Sierra Sciences Identifies 858 Telomerase Inducers
Sierra Sciences [Wikipedia] is also working to develop telomerase activation techiques [Patents]. More than one small-molecule telomerase activator [Links, List] has been found. I list 178 substances under investigation as telomerase activators. Some of them are hTERT transcription activators binding to the hTERT promoter producing hTERT mRNA transcripts and others are hTERT phosphorylation kinases (AKT) that phosphorylate hTERT in the cytoplasm to import it to the cell nucleus. Both seem to produce telomerase activity. In addition, there are HDAC inhibitors like Tricostatin A and CGK 1026 that improve hTERT transcription by expanding chromatin, and cofactors such as HSP90 that are required for hTERT assembly. Sierra Sciences claims to have identified 858 telomerase activity-producing substances after screening 254,593 compounds, more than the 178 telomerase activators I have listed, some of which were identified from Product B. The relative speed with which alternative telomerase activators can be used to achieve rejuvenation is being measured by Sierra Sciences, TA Sciences, Terraternal, RevGenetics, and other firms. For Sierra Sciences (President William H. Andrews PhD) and IsAGenix telomerase inducer test results for 27 phytochemical extracts and associated Product B telomere enlongation results, see the Product B PatentScope.pdf developed by Master Formulator John Anderson. Also see telomerase activator (121) Product B and The Product B Explorer.
Endogenous and Exercise-Induced Telomerase Activators
Note that I have also identified endogenous telomerase activators and 15 exercise-induced telomerase activators together with supplements for promoting their expression. Note that cyclic AMP from exercise and certain supplements such as forskolin can lower caveolin-1 expression in senescent cells by sequestering FOXO factors away from the cellular nucleusin a way that can produce recovery from cellular senescence plus telomere enlongation.
HDAC Inhibitor Telomerase Activators
See papers on telomerase activation, for instance by histone deacetylase inhibitors [Index, Links, Images, Video, Papers, Patents, Books]. "Treatment with tricostatin A (TSA) [Index, Wikipedia, Books, Links, Epigenetic Protocols, chap.8, Future_Medicine/article, article2, anticancer, elevates Hsp22 level with life extending effect] induced significant activation of hTERT mRNA expression and telomerase activity in normal cells, but not in cancer cells." Histone deacetylase inhibitor trichostatin A "activates the hTERT promoter in normal cells", thus enabling telomerase synthesis. I stated above that resveratrol is an activator for SIRT1, a histone deacetylase enzyme [Index/SIRT1, Index/HDACs, Links, Images, Papers, Patents, Books, LifeExtension], the activation of which results in gene silencing that keeps DNA more tightly wound on histone cores, preventing eccDNA formation. Perhaps resveratrol can be used as a SIRT1 gene silencer to shut down telomerase transcription from hTERT following treatment with a small-molecule telomerase activator like tricostatin A(TA/Tricostatin A), a histone deacetylase inhibitor which is genotoxic at some dosages, however. Resveratrol turns out to activate hTERT protein in the cytoplasm via hTERT phosphorylation with the AKT protein kinase (Index/AKT), enabling the import of cytoplasmic hTERT protein into the nucleus, but not improving the number of hTERT mRNA transcripts via activation with a nuclear transcription factor such as HIF-1 or C-myc dimerized with Mad, a helix-loop-helix transcription factor. Both bind to the same type of 5'-CACGTG-3' E-box promoter site on the hTERT promoter. CGK 1026 [Telomerase Activators/CGK 1026, Index, Links, Papers, ChemBank/CGK 1026 molecule], discovered in 2004, is said to "derepress" hTERT expression, and has been used as an orally bioavailable substitute for Tricostatin A. CGK 1026 is thought to inhibit the recruitment of HDAC [Links/HDACs] into E2F-pocket protein complexes assembled on the hTERT promoter. It is described by several sources: Linscott's Directory of Immunological and Biological Reagents, as catalog # 565730, Merck CGK 1026 - Order # 565730-5MG, and EMC Biosciences CGK1026. See also SP1 on the hTERT promoter (hTERT promoter/SP1) for repression and derepression of hTERT transcription. A more detailed look at the situation shows that some commonly available foods contain histone deacetylase inhibitors that may prove valuable in life extension via telomere extension by telomerase activation.
For instance, diallyl sulphide (garlic) and sulphoraphane (broccoli) are zinc-activated (meats, seafood, oysters) class I histone deacetylase inhibitors (HDAC inhibitors). Zinc enhances hTERT transcription by upregulating Bcl-2 (a telomerase activator) and may be used up to 45 mg/day. Another allyl compound from garlic or deodorized garlic [Wikipedia/garlic, Links, LifeExtension] that is more active as a histone deacetylase inhibitor than allyl sulphide [Links] is allyl disulphide [Links], and a still stronger one is allyl mercaptan [Links]. I note that allicinfrom garlic inhibits telomerase expression in cancer cells, often inducing apoptosis. Garlic extends the Hayflick limit in dermal fibroblasts, so that allicin metabolites diallyl disulfide and allyl mercaptan are thought to improve hTERT mRNA expression in normal cells. Note that overdoses of garlic and allyl compounds can have toxic effect; consuming 5 garlic cloves in a tomato soup with chopped onions can induce diarrhea. Butyrate [Index, Links] is also a histone deaceylase inhibitor (10) or chromatin-remodeling factor that may lengthen telomeres by enabling telomerase transcription and is known to extend the life span of Drosophila 40%. Sodium butyrate [Index, Images] is an HDAC inhibitor available now in pill form that may be able to accelerate hTERT transcription by keeping chromatin expanded and available for transcription, so that it might be taken together with astragalosides or other telomerase activators. Sodium butyrate improves the transcription of Sp1, which markedly improves the expression of the telomerase activator epiregulin, a ligand of the epidermal growth factor receptor.
"FR901228" (Romidepsin) is a typical new histone deacetylase inhibitor [Links, LifeExtension] being investigated by Japanese scientists that is known to activate telomerase transcription. Resveratrol (red grapes, Japanese Knotweed root) is a class III histone deacetylase activator via its ability to turn on SIRT1 deacetylase enzyme, collapsing chromatin and inhibiting transcription of hTERT mRNA. However, resveratrol phosphorylates hTERT molecules in the cytoplasm for import into the nucleus, activating telomerase. Resveratrol is sometimes described as a deacetylation activator, and activates the gene SIRT1, yielding the corresponding NAD-dependent SIRT1 deacetylase enzyme for gene silencing via chromatin condensation. NAD, incidentally, is found in tuna, and may be synthesized in the body from niacinamide (nicotinamide), a form of vitamin B3. By 2011 we were using TA Sciences TA-65, astragaloside IV, cycloastragenol, astragalus extract, or astragalus root, in parallel with other telomerase activators [Index, List] such as Nitric Oxide and fenugreek extract to to cover our telomere elongation requirements, with feedback from telomere length testing[LifeXLabs/Telomeasures, Links, Papers, Patents, Books, LEF]. The histone deacetylase inhibitors prepare chromatin DNAfor transcription with RNA polymerase, dissociating the histones from DNA prior to transcription. Some, like sodium butyrate, may be useful for accelerating transcription. There are 4 types of covalent modifications of histone proteins - acetylation, phosphorylation, ADP-ribosylation, and methylation. Histone deacetylation inhibitors allow chromatin to expand and become transcriptionally active, sometimes activating telomerase. Histone deacetylation causes compaction of chromatin, silencing genes. Histone acetylation expands chromatin, while histone deacetylation compacts it.
HDACs may activate p53, while activation of SIRT1 (human SIR2) with resveratrol prevents p53 activation with chromatin compaction and gene silencing, causing extended life span, since telomere shorting itself finally induces a p53-dependent DNA damage detection pathway leading to the senescent state of the cell. Furthermore, resveratrol activates telomerase by phosphorylating cytoplasmic hTERT for import into the nucleus, also tending to inhibit senescence. What we need most are hTERT transcriptional activators that make the correct sections of chromatin transcriptionally active for hTERT mRNA production, such as astragalus root (33 grams/day), astragalus root extract (for application to the scalp), colostrum solution (for transdermal application), hawthorn extract, bacopa (1-2 grams/day), fenugreek seed (3 grams orally before bedtime), arginine (5-10 grams/day for Nitric Oxide), whey protein (for HGH), DHEA (4 x 50 mg/day for higher testosterone), and exercise. HDAC inhibitors assist hTERT transcriptional activators by expanding chromatin and may themselves induce transcription of hTERT mRNA.
Interleukin 2
A larger molecule of about 15,500 Daltons (still capable of passing through the nuclear pore) worth exploring is the cytokineIL-2 or interleukin 2 [Index, TA/Interleukin 2], a "lymphokine" which augments the expression of mRNA for human telomerase in T-cell lymphocytes. IL-2 "has been approved by the Food and Drug Administration (FDA) for the treatment of cancers (malignant melanoma, renal cell cancer), and is in clinical trials for the treatment of chronic viral infections, and as a booster (adjuvant) for vaccines." - [Wikipedia]. Interleukin 2's telomerase-enhancing role in T-lymphocytes has been noted by other investigators. [See IL-2 source BD, Links/IL-2]. There is evidence that DHEA acts as a physiological regulator of interleukin-2 biosynthesis (Yu, 1995, citing in Arking, The Biology of Aging, p.233.), so supplementation with DHEA may help interleukin-2 maintain T-lymphocyte telomeres.
Estrogens
Preliminary data suggest estrogen [Telomerase Activators/Estrogen] activates telomerase in T-lymphocytes [Handbook of Models for Human Aging, p.37]. Furthermore, "estrogen and its receptor activate telomerase in estrogen-responsive cells through the estrogen response element sites in the hTERT promoter".
Epithalon Peptide
Another group in Russia at the St. Petersburg Institute of Bioregulation and Gerontology announced in 2003 that Epithalon Peptide [Index, Telomerase Activators/Epithalon Peptide, epitalon, aka epitalon], a small 4-peptide protein made from the sequence alanine, glutamine, aspartic acid, and glycine, (Ala-Glu-Asp-Gly), activates telomerase and extends telomeres [Links, Papers, Original Paper, Biogerontology article 1, article 2, epithalamin articles, sources, sources2, sources and costs]. Epithalon peptide has been injected 5 times weekly into mice, resulting in 34.2% prolongation of life span in mice without cancers. Liposomal or sublingual (under the tongue) administration is probably possible with such short peptide molecules, and perhaps it can be topically applied like a skin cream, taken sublingually under the tongue, in buccal fashion in the pouch of the cheek, or otherwise.
HGH and IGF-1 as Telomerase Activators
Mice overexpressing IGF-1 showed higher levels of phospho-Akt [Links, Books] leading to increased telomerase activityand more effective cardiac stem cells, creating a better supply of new myocytes for the heart. (Torella et.al., 2004, cited by Arking). Telomerase activity can be stimulated by improving our levels of IGF-1 (Telomerase Activators/IGF-1) by direct input of the hormone or by stimulating it via exercise in the presence of amino acid stacks [Human IGF-1 stimulator, Links/IGF-1, Books].
I note that Human Growth Hormone (HGH) has been shown to upregulate telomerase in ovaries and in liver cells (hepatocytes). [Links, Books]. Furthermore, HGH (which improves transcripiton of hTERT mRNA) is partially converted by the liver into IGF-1 (which phosphorylates hTERT protein for import into the nucleus). Note that HGH and IGF-1 are both upregulated by exercise, as are at least 12 other endogenous telomerase activators and at least 15 telomerase-activating human growth factors found in growth factor skin creams. On the other hand, an antagonist of growth hormone-releasing hormone has dramatically decreased telomerase activity [Links, Papers, Books, Books2] in experimental xenografted transplants [article]. Telomerase activation has been achieved in cord blood using IGF-1 in the presence of PHA (phytohaemagglutinin; 1 μg/ml, Sigma, St Louis, Mo., U.S.A.). Note that while attempting telomere extension via a growth hormone strategy for telomerase activation [Patents], one should avoid ingesting telomerase inhibitors, which can sometimes be produced in the body from high-polyphenol diets. However, some telomerase activators for normal cells (such as silymarin) behave like telomerase inhibitors when applied to cancer cells.
Ecdysone
Evidently, there has also been some progress in hTERT activation using the insect hormone ecdysone (TA/Ecdysone, muristerone) to induce transcription from hTERT and cellular immortalization in human fibroblasts [article]. Finally, I note that US Patent 6787133 has been granted to Geron for a procedure to purify telomerase for the purpose of identifying telomerase activators and telomerase inhibitors.
Increased telomere length, more telomerase activity, and active meristematic stem cell presence have been identified as important factors in the long life spans of 2000-5000 year old bristlecone pine trees [106]. Furthermore, inducing more telomerase activity in embryonic stem cells seems to improve their defenses. Although telomerase expression is up-regulated in cancer, and used to detect cancer, the gene for telomerase is not an oncogene and its activation does not induce cancerous growth deregulation by itself. Inhibition of telomerase in human tissues helps suppress run-away cancer cells by inducing massive apoptosis in tumors, so telomerase activation treatments to lengthen our telomeres for life extension purposes [Age Transformation, Cyclic Telomerase Activation] should be periodic and short-term, or done ex vivo prior to an adult stem cell transplant, and not continuously in vivo. Telomerase activators are weakly tumor promoters, as they inhibit telomerase inhibition as an anticancer defense. TA Sciences proposes using their TA-65 telomerase activator 3 months on, 3 months off, and 3 months on again for a year (the Patton Protocol).
Cyclic Telomerase Activation with Readily Available Nutraceuticals
I expect that monthly cycles of 2 weeks of telomerase activation followed by two weeks of telomerase inhibition plus treatment with anticancer nutraceuticals is an adequately safe approach. This may allow stronger telomerase activation than is feasible with the Patton Protocol. In the future we may prefer to use Product B, astragalus root extract with chitosan, purslane extract, black cohosh, colostrum (for telomerase-activating growth factors and IGF-1), ginkgo biloba for HIF-1, fenugreek extract (for HIF-1, testosterone, diosgenin, and insulin), arginine for Nitric Oxide, alpha-GPC with exercise for HGH, Nerve Growth Factor boosters (acetyl L-carnitine, huperzine A, carnosic acid from rosemary) to promote ID-1 helix-loop-helix transcription factor, or other telomerase activators under investigation to lengthen our telomeres, along with telomerase-activating foods like whey protein (for HGH), creatine monohydrate (for IGF-1 and to boost cAMP), apples and onions (for quercetin), and garlic (for HDAC inhibitors improving hTERT mRNA expression). Some of these, such as astragalus root extract, colostrum, and black cohosh, may be taken orally and also rubbed in to the skin and scalp directly for best effect, rubbed into the gums and oral cavity, or even applied in suppository form (astragalus extract in glycerin). Telomerase-activating drops for the eyes (similar to carnosine drops for the eyes) should be researched. Perhaps colostrum spray mist would be suitable, although it must be refrigerated and verified as useful and safe. Essential oil of rosemary may have telomerase-activating effect in the sinuses via nerve growth factor activation of ID-1, where we might also consider using colostrum spray mist or astragalus extract nasal spays.
Hilariously, no mouse has ever died from telemere shortening, or suffered from a shortage of telomerase, however. In mice the dominant aging mechanism seems to be mitochondrial aging via lipid peroxidation.
On the other hand, relatively cancer-resistant humans can die from telomere shortening; as telomere length goes to zero once-mitotic bone marrow, human skin, liver, colon mucosa and lymphocyte cells become senescent and chromosomally unstable. Lymphocytes then suffer irreversible cell cycle arrest. Cell death follows, sometimes after a lengthy delay. Progress on small molecules for telomerase activation is also proceeding from research on plants. Recently it has been shown that telomere shortening is aggravated in each cell division of mitotic cells by high homocysteine levels, so that a homocysteine shield [Links, Images] featuring TMG (trimethyglycine) 500 Mg - 720 mg, folic acid 400 Mcg, vitamin B6perhaps 25 Mg, and vitamin B12 250 Mcg is effective at preserving telomere length, in addition to preserving the endothelial cell linings of the veins and arteries of the heart and the brain and fending off atherosclerosis. [See Dr. Phillip Lee Miller, Life Extension Revolution.] Both antioxidant treatment preventing free-radical-induced single-strand DNA breaks (repaired by Cat's Claw extract (AC-11) [Index] along with double-strand breaks and photochemical DNA damage) and homocysteine treatment featuring a homocysteine protection formula preventing more telomere loss per cell division tend to preserve telomere length, so that some centenarians seem to have rather long telomeres.
Another possibility for lengthening telomeres, Alternative Lengthing of Telomeres [Links/ALT], may be implemented with DNA nanocircles, which seem to be generated in cancer cells from long telomeres via recombination of long telomeres achieved by homologous DNA repair. Researchers picture the small telomeric circles forming through the resolution of an intratelomeric strand invasion resembling a t-loop during homology-directed repair (HDR) of telomeric DNA usually repressed by the POT1 component of the telomere nucleoprotein complex shelterin, also termed the telosome. Two recent papers demonstrated that human alternative lengthening of telomere (ALT) cells have abundant t-circles similar to Telomolecular Corporation's (Corporate Video) DNA nanocircles, pointing to their potential role in promoting telomere replication in the absence of telomerase. "Analysis of telomere restriction fragments from human cells that rely on ALT for telomere maintenance revealed that they possess telomeric tracts that are extremely heterogeneous in length, ranging from undetectable to abnormally long (Bryan et al., 1995), which would also point towards a recombinational origin (see also Henson et al., 2002)." Intense interest in the ALT pathway exists partly because it is a stumbling block to the application of telomerase inhibition as cancer therapy. Without telomere extension via small molecule telomerase activators or synthetic nanocircle ALT, however, it seems death is finally certain, as telomeres will be consumed eventually otherwise, destroying T-lymphocytes in the immune system, microglial cells in the brain associated with clean-up operations, the skin, the lining of the internal organs, and all other mitotic cells including the lining of the colon.
I might add that the consequence of cloning an animal from a mature specimen with shorter-than-embryonic telomeres is that the clone dies young, as in the case of Dolly the sheep, the first cloned mammal to make headlines. To successfully clone an adult requires pre-extension of telomeres prior to nuclear transfer or cloning from embryonic tissues, otherwise, the cloned offspring may die ahead of time [Books]. In practice, cattle are typically cloned from embryonic or fetal cells to avoid the short-telomere early death syndrome. However, recently we have found out that cloning from senescent cells at the end of their lifespan can produce strong telomerase activation in a way that produces potentially very long-lived offspring in cattle with telomeres longer than ever! It is also noteworthy that people having a longer telomere length (upper half of the population) live longer than those in the lower half of the population, who have 1.86 times the mortality rate, with 3.2 times the heart disease and 8.5 times the infectious disease of the long telomere upper half. [Cawthon, et.al., 2003, cited in The Biology of Aging by Robert Arking, p.442.]
Breast Cancer, Cell Division, and Astragalus Root
The average age for breast cancer in males is 67, and in women 62, afflicting 1 in 8 women over a lifetime. During each menstrual cycle, estrogen together with other ovarian hormones signals cells in the breast to divide and multiply. Breast enlargement involving estrogens and other ovarian hormones in males or females may result in cell divisions sufficient to drive cells closer to the Hayflick limit, when they become senescent and genomically unstable after telomeres become uncapped, thus cancer-prone. Thus anti-senescence drugs like the small-molecule telomerase activators in astragalus root extracts or silymarin may be useful in preventing breast cancer arising from genomic instabilities in breast cells that have divided many times and eventually become senescent ahead of other cells as a consequence of estrogen and ovarian hormone signaling. I note that telomerase activator black cohosh improves the stability of breast tissue against cancer. Busty aging mates might take astragalus root extract to ward off breast cancer before it is observed, it seems.
We wonder if the term "astragalus" itself did not signify originally this set of patients that could benefit from the use of the herb. "Recent studies show that more than 90% of all cancer is caused by critical telomere shortening [Links, Images, Papers, Books; Links/cancer due to critical telomere shortening, Books], for example, in 97% of premalignant epithelial lesions [Images] critical telomere shortening is observed (Meeker, John Hopkins University 2005, Papers)." - Telomolecular Corporation SEC statement. In the breast, progesterone also acts as a chemical messenger that tells breast cells to divide, hence progesterone and estrogen are applied in female transformations which can become ultimately dangerous when finally cells approach the Hayflick cell division limit. [Links/breast cancer, Books, Wikipedia, Index]. Chromosome 17 has short telomeres [Links] implicated in breast cancer. Perhaps astragalus extract in glycerin or in liposomes would be useful if applied topically to the breast, in addition to astragalosides or other small molecule telomerase activators such as black cohosh taken orally to apply telomerase activation in a cyclic manner for improving stem cell and tissue genomic stabilityagainst malignant transformations.
The Telomere Positioning Effect & Youthful Patterns of Gene Expression
Also see the telomere positioning effect [Links, Images, Papers, Patents, Books, LifeExtension]. Long telomeres silence genes close to the telomere, and as the telomere shrinks genes proximate to the telomere are more expressed. Therefore to obtain youthful patterns of gene expression in mitotic, dividing cells, it is desirable to maintain long telomeres [Papers, Patents Books]. Telomeres are transcribed to produce TERRA telomeric RNA, starting from subtelomeric DNA and moving toward the end of the chromosome, when telomeres become uncapped. This reduces the telomere position effect inhibition of the transcription of genes near the telomere, and may improve telomeric DNA repair, since some DNA repair is only done during transcription. A good deal of work is now going on to identify telomere-shortening-sensitive-genes (TSSG) [Links, Papers, Patents, Books]. I note that the shortest telomere on human chromosome 17p [Books, Wikipedia/Chromosome 17, Ornl, Genes & Disease; GeneCards/P53] at 17p13.1 seems to be fairly close (within 7,512,445 bp out of 80 million bp) to the gene for the tumor suppressor gene p53, so that more tumor suppressor p53 protein should produced when this telomere gets sufficiently short. This way shorter telomeres would work together with the tumor-suppression system and apparatus for stopping the cell cycle and to induce the senescent state of the cell when telomeres shorten. See Links/the telomere position effect and tumor suppressor gene p53. "The level of p53 protein increases in near senescent cultures" of cells [Vaziri and Benchimol, 1996].
It turns out that subtelomeric DNA contains genes for zinc fingers [ZFC, Wikipedia, Links/zinc fingers, Books] and olfactory receptors [Books] that should be more actively transcribed when telomeres are short. Zinc fingers [Books, Books/zinc fingers in cellular senescence] may be transcribed in clusters that activate or deactivate promotors for genes like fingers applied to a piano keyboard. Their telomere position effect activation in subtelomeric DNA when telomeres become short may directly impact gene expression to produce some of the observed aging effects. Perhaps someday RNA interference [Images, Papers, Patents, Books], or RNAi, may be applied to oppose undesirable effects caused by the expression of mRNA controlled by zinc fingers in subtelomeric DNA. Genes involved in the wrinkled skin, graying hair, and healing abilities may be involved, as Geron has shown that these are improved by lengthening telomeres [Wikipedia/Telomerase]. "Cells with sufficiently elongated telomeres energetically produce, in high levels, proteins like catalase, superoxide dismutase, glutathione, Ku, collagen, elastin and many other proteins important in tissue formation, cell repair, and antioxidation, that become scarce as telomeres shorten." [From Telomolecular Corp/case studies, see also LifeExtension/Telomere Control and Cellular Aging, the papers of Dr. Woodring Wright and Dr. Jerry Shay.]
"As the hTERT gene is only a few hundred kilobases from the end of chromosome 5p [Books], one could speculate that TPE (silencing) of hTERT limits the maximal length of human telomeres during embryogenesis." [JW Shay & WE Wright, 2005.]. (In earlier 2002 work [Cong, Shay, and Wright, 2002], the hTERT gene was stated to be the most distal gene on 5p, but 2 million base pairs from the telomere, so that the telomere position effect would have little effect on hTERT.) Evidently, the telomere position effect should cause the body to automatically resist aging by more strongly activating telomerase as we age, a tendency we must supplement to achieve a longer-than-usual lifespan. Perhaps proteins mediating telomerase assembly such as p23 and Hsp90 are less available as time goes on. The location of the hTERT gene (TERT) in other species with different lifespans becomes an interesting question. Species may be engineered in the future with extra distal hTERT genes on other chromosomes to extend their life spans. Perhaps the telomere position effect is also involved in thymic recovery and the withering of the thymus gland, which is involved in T-lymphocyte production and connected to the immunological theory of aging (11). Recovery of thymus gland function is sometimes observed when a patient is treated with 10 grams of arginine per day to produce Nitric Oxide, a telomerase-activating vasodilator.
On the other hand, as we shall see according to the Membrane Hypothesis of Aging (13), to obtain youthful patterns of gene expression may also require modifying the cell membrane permeabilities (modified by cross-linking of membrane-imbedded proteins) to ionic species such as potassium that modify the dehydration, colloidal properties, and density of cellular material, factors which should apply to both mitotic and non-mitotic cells. In dense, dehydrated cells enzyme reactions are inhibited, so that protein turnover and protein synthesis are retarded, tending to produce more ceroid wastes and lipofuscinthat choke the cell and increase free radical production, eventually leading to inflammation and processes involving proinflammatory cytokines that are ultimately poisonous to the cell. Also, deproteinization of chromatin restores its youthful low DNA strand separation temperature, so that restoring youthful patterns of gene expression [Books] may be assisted by undoing protein cross-links or other protein properties in chromatin that inhibit DNA strand separation [Books] and gene expression [Books, Books/chromatin and gene expression]. See Maria A. Blasco on the telomere position effect in Maria A. Blasco (2007), The epigenetic regulation of mammalian telomeres, Nature, April 2007.
Telomere Capping Proteins
[Index/Telomere Loop Control Proteins, Index/Mammalian Telomere Protein Factors, Telomere Extension by Telomerase, Telomerase Components in Cell Signaling, Links, Papers, Books, Links/capping supplements].
The telomere t-loop may be opened to make it accessible to telomerase by phosphorylating tankyrase 1 with insulin using insulin-boosters such as Gymnema Sylvestre, Fenugreek Extract, Fenugreek seeds, or 4-hydroxyisoleucine. This may be amplified somewhat in the presence of dextrose. Tankyrase 1 levels may also be boosted with niacinamide (nicotinamide, a form of vitamin B3). Tankyrase 1 uses NAD as a substrate in poly(ADP-ribosylation) of TRF1, so NAD supplements[Links] may be useful when applying tankyrase 1 to enable telomerase access to the end points of otherwise closed telomere t-loops. Note that nicotinamide elevates NAD+ (NADH, Nicotinamide Adenine Dinucleotide) levels, but should be taken early in the morning on telomerase activation days to build up NADH levels, since it has inhibiting effect on PARP enzymes such as tankyrase 1 that I prefer to phosphorylate via evening doses of insulin-boosters such as Fenugreek extract. Tankyrase 1 opens the telomere t-loop via telomeric PARP activity involving poly(ADP-ribo)sylation with a NAD+ substrate that strips the telomere of the telomere binding protein TRF1. Once the loop is open, the telomerase holoenzyme can access the telomere to extend it.
Uncapped telomeres [Links, Images, Papers, Patents, Books] terminate in a single-stranded overhang 100-300 nucleotides in length bound to the telomere loop along its length by POT1 and at its starting point by TRF1. Dr. Yie Liu's lab at the National Laboratory of Molecular Gerontology has illustrated that the proteins PARP1 [Index, Links, Images, Papers, Patents, Books] and MSH2 [Index, Links, Papers], a DNA mismatch repair protein, play a role in maintaining telomere capping function [Links, Images, Papers, Patents, Books] in which a telomere t-loop configuration many thousands of base pairs long is believed to protect chromosome ends from being recognized as broken DNA. As I note in section 10 on DNA Repair, the maximum life span of 13 species is directly correlated to the activity of poly (ADP-ribose) polymerase (PARP) in mononuclear leukocytes. Disruption of the telomere loop [Images Papers, Patents, Books] and subsequent exposure of the 3' overhang represents the uncapped state of telomeres. The ability to mask telomeres from being recognized as damaged DNA is crucial to maintaining normal cellular function, as uncapped telomeres directly associate with many DNA damage response proteins [Links, Images, Papers, Patents, Books] in telomere-initiated cellular senescence. Telomere integrity depends on the ability to maintain telomere length and/or the ability to mask telomeres from being recognized as damaged DNA via telomere capping [Links, Images, Papers, Patents, Books].
Statins [Links, Images, Papers, Patents, Books] have been observed to provide an enhancement of the associated telomere protection biology [Paper, Books], but are often avoided due to certain hazards. In certain experiments atorvastatin (0.1 mol/L) and mevastatin (1.0 mol/L) both led to a more than 3-fold increase in the expression of the telomere capping protein TRF2 (telomere repeat-binding factor, Index/Telosome), as shown by immunoblotting. Simvastatin (Zocor) is even more effective in promoting TRF2. Today atorvastatin is available by prescription, but mevastatin is not used due to multiple side effects. Uncapping of telomeres may be detected by the loss of TRF2, and the effect of the statin drugs has been produced by application of exogenous TRF2 [Index/TRF2, Papers, Books], which protects human telomeres from end-to-end fusions. TRF2 binds to double-stranded telomeric DNA. PARP1 interacts with TRF2 and is involved in repairing damaged telomeres. "As the length of telomeres in leukocytes shortened the risk of coronary heart disease increased... the risk was substantially attenuated by pravastatin [Links, Books]. Since statins have been shown to increase the production of a protein-telomere capping protein that prevents telomeres from shortening it is a potential hypothesis that statins bring about some benefits by this mechanism. This is very early research but it implies that statins could well have the ability to slow certain aging processes." - ErinPharm Gazette, Jan. 2007.
I note that my friends have warned me not to meddle with statin drugs due to side effects [article, Books, Links] such as lowering the body's level of CoQ10. Furthermore, work by Woodring E. Wright and Jerry W. Shay showed that TRF2 overexpression can degrade telomeres. Effective treatment to improve levels of telomere-capping associated proteins is, however, definitely on the horizon. Overexpression of TRF2, which stabilizes the telomeric loop, shifts the telomere length threshold for senescence towards shorter telomeres (Karlsreder et al. 2002), so that TRF2 overexpression has been observed to delay senescence. Furthermore, TRF2 overexpression improves double-strand DNA repair (Mao et al. 2007). Recent research shows that TRF2 is a factor in telomere length regulation, and in preventing telomere fusion between chromosomes (Smogorzewska et al. 2000, van Steensel et al. 1998, Celli and de Lange 2005). By 2011, however, there were more indications that TRF2 overexpressionmay be dangerous (M.A.Blasco, 2008, in K. Lenhard Rudolf, 2008, p.283). TRF2 is upregulated in some human carcinomas, along with TRF1 and TIN2 (Oh et al. 2005, Matsutani et al. 2001, Munoz et al. 2005, Nakanishi et al. 2003, Bellon et al. 2006). TRF2 overexpressing mice show increases in chromosomal instability and telomere recombination(Munoz et al. 2005, Blanco et al. 2007) and also rapid and dramatic loss of telomere sequences where skin is exposed to light (Munoz et al. 2005).
Also note that expression of telomerase protects the telomere cap, so that small-molecule telomerase activators such as astragalus extract, astragaloside IV, or cycloastragenol defend telomere capping. Telomere uncapping induces the state of cellular growth arrest termed senescence, or mortality state M1. [Senescence and Immortalization: The Role of Telomeres and Telomerase, Jerry W. Shay and Woodring E. Wright, from Carcinogenesis, 2005]. If the genes maintaining the senescence checkpoint M1 are blocked, say by a viral oncogene, the cells continue to divide and proceed to the crisis state M2, typically followed by cellular apoptosis. Both M1 and M2 can be prevented by introducing telomerase. [Telomere Regulation in Eucaryotic Cells, from Chromosomal Instability and Aging: Basic Science and Clinical Implications By Fuki M. Hisama, Sherman M. Weissman, George M. Martin, Informa Health Care, 2003.] The telomere t-loop closes closer to non-canonical sequences differing from GGTTAG repeat (subtelomeric repeats) as cell divisions proceed and the telomere shortens. The telomeres are reduced by 50-200 bp per division until the telomere tail fails to close into a t-loop(bound with a small d-loop) when TRF2 cannot find a proper binding site due to the non-canonical nature of the repeat there, for instance and typically GGTGAG.
See also Carol W. Greider's Telomeres Do D-Loop T-Loop Minireview, IMGENEX on telomere-associated factors, Jerry Shay's turn-of-the-century article in Nature on on telomeric t-loops and d-loops, Dr. Connie Nugent's lab, and Joao Pedro de Magalhaes on telomeres and telomerase. Recent work shows that telomere-protection protein POT1 is involved in binding the single-stranded overhang at the tip end of the telomere to close the t-loop and is required for telomere length regulation and chromosomal end protection [Yang, et.al, 2007]. Humans have one POT gene, mice have two. Telomeres are bound to a set of numerous interacting proteins [Index] including 6 shelterin subunitsRAP1, TRF1, TRF2, TIN2, TPP1, and POT1, which insure proper maintenance of telomeres [Index]. It has been described as the telomere binding complex [Links, Images Papers, Patents, Books], the telosome [Links, Images, Papers, Books], or more recently shelterin [Links, Images, Papers]. Note that telomeric protein complexes are also being investigated by Dr. Zhou Songyang [Papers, Papers/telomeres and Dr. Zhou Songyang]. Jesse Fender and other investigators including Woodring E. Wright and Jerry W. Shay have been exploring the interaction of the protein Ku and telomerase. Ku (KU70, KU86), which is involved in localizing telomeres to the nuclear membrane, is also important in assisting telomerase function, lack of Ku leading to telomere-telomere fusions and cellular dysfunction. For epigenetic factors in telomere lengthening and corresponding therapeutic possibilities, see Telomeric Chromatin.
Blackburn: Telomerase Does Not Act on Capped Telomeres
Incidentally, according to a model propounded by E. Blackburn in Switching and Signaling at the Telomere (2001), telomerase does not act on capped telomeres, but only on telomeres that have become short enough to become uncapped. Telomere enlongation via telomerase then provides telomeres long enough to cap themselves. This seems to be supported by TA-65 testing in 2010. See Calvin B. Harley, Weimin Liu, Maria Blasco, Elsa Vera, William H. Andrews, Laura A. Briggs, and Joseph M. Raffaele (2010), A Natural Product Telomerase Activator As Part of a Health Maintenance Program, Rejuvenation Research, September 7, 2010. However, telomerase activation can easily result in telomere growth > 55 bp/year. Retinal pigment epithelial cells transfected with hTERT plasmids show telomeres lengthening at 115-255 bp per population doubling, and we find that BJ foreskin fibroblasts similarly transfected show a telomere length increase of 340-370 bp per population doubling, the same order of magnitude (400-460 bp/year telomere growth, corresponding to roughly 1 cell division per year) originally observed at TA Sciences in 2007 by Bob Waskom and Greta Blackburn. - From Andrea G. Bodnar, Michel Ouellette, Maria Frolkis, Shawn E. Holt, Choy-Pik Chiu, Gregg B. Morin, Calvin B. Harley, Jerry W. Shay, Serge Lichtsteiner, and Woodring E. Wright (1998), Extension of Life-Span by Introduction of Telomerase into Normal Human Cells, Science, vol. 279, 16 January 1998. Normally, human fibroblasts can divide for about 49 population doublings.
"Human fibroblasts expressing the catalytic component of human telomerase (hTERT) have been followed for 250–400 population doublings. As expected, telomerase activity declined in long term culture of stable transfectants. Surprisingly, however, clones with average telomere lengths several kilobases shorter than those of senescent parental cells continued to proliferate. Although the longest telomeres shortened, the size of the shortest telomeres was maintained. Cells with subsenescent telomere lengths proliferated for an additional 20 doublings after inhibiting telomerase activity with a dominant-negative hTERT mutant. These results indicate that, under conditions of limiting telomerase activity, cis-acting signals may recruit telomerase to act on the shortest telomeres..." from Michel M. Ouellette, Martha Lia, Brittney-Shea Herbert, Mari Johnson, Shawn E. Holt, Heidi S. Liss, Jerry W. Shay and Woodring E. Wright (2000), Subsenescent Telomere Lengths in Fibroblasts Immortalized by Limiting Amounts of Telomerase, The Journal of Biological Chemistry, April 7, 2000, vol. 275, 10072-10076. See also telomerase artist's conceptions [telomerase & proteins database, telomere image]. Note that telomerase inhibitor RHPS4 [TI/RHPS4, article] acts through stabilization of four-stranded G-quadruplex structures formed by single-stranded telomeric DNA found at the end of the t-loop structure, so that telomerase does not act on the closed telomere loop. I get the impression that the percentage of senescent cells in a tissue determines how old it looks, so that the macroscale human phenotype can be rejuvenated by merely making telomeres long enough to close the t-loops, as far as mitotic cells are concerned.
However, recovery from replicative senescence can be frustrated by high levels of P16INK4A, which is down-regulated by the Id-1 helix-loop-helix transcription factor, itself upregulated by Nerve Growth Factor (NGF), upregulated by acetyl L-carnitine, rosemary tea, and other nutraceuticals. P16INK4A can also be reduced by exercise, carrots, and retinol.
It is certainly true that maintaining long telomeres supports closed-loop telomere structure that frustrates the arrival of replicative senescence, especially in the absence of oxidative stress leading to premature stress-induced senescence. The best way to to keep telomere loops lengthy is probably to use telomerase activators, which have closure possibilities for telomere t-loops to help cells rejuvenate and avoid replicative senescence. However, as cells accumlate more and more P16INK4A, recovery from the state of replicative senescence becomes more difficult. Eventually, perhaps a healthy adult stem cell with vigorously long telomeres can fill a spot where a senescent cell has become apoptotic in such cases. In time, rejuvenated cells secrete collagen, elastin, and endogenous antioxidants in a way that restores a youthful look, although ceroid or lipofuscin deposits may have to be removed with CoQ10, alpha lipoic acid, Piracetam, or other drugs to completely finish the job. Note that collagen and elastin can both be restored in the extracellular matrix by telomerase-activating colostrum skin creams or by telomerase-inhibiting TGF-beta skin creams. There may be milage in unlocking closed telomeric loops for telomerase activation, then resealing them, to achieve even longer telomeres for a still more youthful patterns of gene expression associated with longer telomeres. Can telomeres be made as long as desired by the continued application of telomerase activation? Or does everything stop when the t-loop closes and the cell assumes the immortal phenotype? It is certain that continued application of telomerase activation can supply hundreds of additional cell divisions, and that immortal cells continue to divide in a way that maintains fairly constant or increasing telomere length.
Atorvastatin (Lipitor), Simvastatin (Zocor), TRF2, T-Loop Closure, and Rejuvenation
Theoretically, Lipitor (atorvastatin) should be life-extending via its activation of telomere binding protein TRF2. Applying Lipitor at the M1 state with 4000 remaining base pairs might yield about 4000/50 = 80 further cell divisions and lifetimes on the order of 220 years. The additional cell division capacity should strengthen the immune system. The closure of telomere t-loops by TRF2 should remove senescence effects. It could be that Lipitor (atorvastatin) is more rapidly rejuvenating than anything else we have at just $65/month for the prescription drug. A cheaper version, lovastatin, is available, and Zocor (simvastatin) is more powerful. Also check pravastatin, which should produce similar results. Extra CoQ10 or ubiquinolshould be taken, along with a homocysteine shield and antioxidants, including mitochodrially effective antioxidants like alpha lipoic acid and protective drugs like acetyl L-carnitine. Perhaps the most effective program for antiaging will begin with atorvastatin and end with cycloastragenol/resveratrol cycles. This analysis, however, needs experimental verification and requires more study, including side-effects analysis. Some studies maintain TRF2 overexpression damages telomeres, however, so TRF2 overexpression is unlikely to become a preferred life extension strategy.
Telomere Enlongation with Replication Protein A and hEST1A
Other control proteins associated with telomere maintenance include Replication Protein A and hEST1A. Replication Protein A on human Chromosome 17 [Index, Links, GeneCards/RPA1, (YeastGenome/RPA1, YeastGenome/Telomere Maintenance)], which is present in all eucaryotic cells, has been observed to activate telomerase in yeast. See Vera Schramke, Pierre Luciano, Vanessa Brevet, Sylvine Guillot, Yves Corda, Maria Pia Longhese, Eric Gilson & Vincent Géli, (2003, 2004), RPA regulates telomerase action by providing Est1p access to chromosome ends, Nature Genetics 36, 46 - 54 (2004) Published online: 21 December 2003. Estp1 [YeastGenome/Est1] has been conserved in evolution and is present in humans as hEST1A [Links]. "Overproduction of hEST1A cooperated with hTERT to lengthen telomeres." - See Snow BE, Erdmann N, Cruickshank J, Goldman H, Gill RM, Robinson MO, Harrington L., (2003), Functional conservation of the telomerase protein Est1p in humans, Current Biology 2003 Apr 15;13(8):698-704. hEST1A recruits telomerase holoenzymeby binding to hTERT. (See Diagram). hSMG6 [Links/hSMG6, Links/SMG6] is identical to hEST1A. (See Lingner Joachim, Senior scientist, Telomerase and chromosome end replication, ISREC.) According to Gene Cards, overexpression of SMG6 (in humans hEST1A) results in telomere uncapping. Therefore perhaps hSMG6 or hEST1A can be used to extend t-loop capped telomeres, opening them so that they can be extended by telomerase. Telomerase does not act on telomeres unless the telomere is uncapped, as we saw in the last paragraph. Thus large telomere t-loops corresponding to very youthful cells may be prepared by using hEST1a (hSMG6) together with telomerase, probably by stopping SMG6 towards the end of a treatment period to reseal telomere t-loops. Such cells may have superior staying power, lasting for more cell divisions before uncapping, generating a DNA damage signal, and entering the state of replicative senescence.
"TA-65 alone will reduce the percentage of cells with short telomeres ( < 4 kbp) with minimal effects on mean telomere length..." - Calvin B. Harley, et.al, 2010. Perhaps other telomerase activators will turn out to gradually reduce the percentage of short telomeres ( < 4kb) without modifying average telomere length. To improve on average telomere length much with telomerase activation may require activation of hEST1A and Replication Protein A, so that t-loops can open for telomerase access.
The Telomere DNA Helicases and Co-Factor Magnesium
The WRNp helicase protein (associated with Werner Syndrome) that unwinds DNA turns out to be complexed on the telomere [Image] with other t-loop proteins [Index]. The proper function of WRNp requires Mg2++ as a cofactor, so that telomere maintenance may be improved by taking magnesium supplements. Cells lacking WRN helicase activity show defective telomere lagging strand synthesis [Crabbe, et al, 2004]. Life Extension Magazine has noted that low magnesium levels common in the population lead to high rates of hypertension and sudden death. This is perhaps partially due to premature endothelial senescence linked to WRNp helicase malfunction from Mg2++ cofactor shortfall and associated with high monocyte adhesion and consequent formation of atherosclerotic plaque. The magnesium ion is also an essential cofactor for DNA polymerase whenever human DNA is synthesized without a reverse transciptase like telomerase. This was discovered by Arthur Kornberg in the 1960s. (Magnesium is also a cofactor for RNA polymerases.) Note that magnesium supplementation may be used to decrease high blood pressure in non-senescent cases, lowering the risk of heart attack and stroke.
The Time Machine Effect
[agetransformation.html, lifexnotes2.html/TIME MACHINE]
Cosmic Humor and Pursuit of the Aquarian Soul: Moonwalking Scattered Backwards in Time
By watching Indra became
King of the Gods.
How wonderful to watch.
How foolish to sleep.
- Green and the Great Religions.
Keratinocytes of the epidermis live about as long as the period of the moon by transiently expressing telomerase, showing perhaps 1000 stem cell divisions over a lifetime, and coincidentally the synced-up moon projects the mythos of shedding one's "skin". The book of Proclus on the moon-god's spine is thus a "telomere" at the end of the line on the moon, a haunting "God Particle" or "M.Fossel". Furthermore, the Hayflick and Moorhead revolution of 1961 may be mirrored at the summit of NGC 2264 as our hero makes his way to eternal glory as Orion amid his amazing Winter Crescent flying saucer of stars, shining on the smile of Andromeda in Perseus, the Pleiades, and Aries above like the first light of the Sun on the bottom of the New Moon.
Rejuvenation Rates
Note that TA Sciences, using astragalus extract TA-41 in 2005 on a 3-month-on, 3-month-off cycle for a year measured 460 bp/year telomere growth in blood granulocytes, while aging normally subtracts about 50 bp/year, so that with TA-41 the telomere biotimer moved backwards in time about 9 times as fast as it went forwards when "aging", after normal aging base pair losses were accounted for. That is, we observed a rejuvenation rate of about 9 years per year, or 0.75 years/month. Adults may experience 1 to 2 cell divisions per year, since approximately 50 cell divisions take place from the embryonic stage of development in mitotically active human somatic tissues excepting stem cells and germ cells. Persistence is required in de-aging using telomerase activators, as the process takes place gradually, somewhat like aging itself. However, instead of merely slowing the aging process, telomere remodeling with cyclic telomerase activation is expected to carry its practitioners all the way back to the young adult state, if telomeres measure longer year by year. Bear in mind that antioxidants and antiglycating drugs, DNA repair acceleration, homocysteine screens, cell membrane treatments, and vitamin C should help ensure a successful outcome with a minimum population of senescent cells to threaten cancer or susceptibility to diseases of old age. Note that there are 10 bp/turn in Watson-Crick B-DNA, with 33.2 Angstrom units per turn, or 3.32 nm/turn, so that in one year when 50 bp are lost, we typically lose 16.6 nm of telomere length. On the other hand, using TA-41 according to the Patton Protocol, we should see 9 x 16.6 nm = 149.4 nm of telomere growth per year, amounting to 1494/33.2 = 45 turns of the Watson-Crick DNA double helix at each end of every chromosome, about 76.67 telomeric GGTTAG satellite DNA repeats/year. 149.4 nm is in the EUV extreme ulraviolet range of wavelengths, and more than 2 years of growth are required for the telomere growth length to approach the wavelength of violet light, 380-450 nm. I note TA Sciences TA-41 user Bob Waskom, aged 69, observed a rejuvenation rate of B = By = -8 years per year, based on 400 bp of telomere growth per year, which amounts a to a Bm = - 0.667 years/month rejuvenation scenario. See TA Sciences testamonials.
More recent studies of TA-65 (Calvin B. Harley, et. al., 2011) do not show such dramatic lengthening of telomeres, but rather closing of the shortest telomere t-loop to prevent cellular senescence. It will be useful to measure the rejuvenation rate constant B in years/year for telomerase activators in general, in parallel, in the presence of transcription accelerators, and while on specific diets targeted to improve by reducing the intake or expression of telomerase inhibitors. For instance, it will be useful to test B in connection with schemes for improving the rejuvenation rate such as adding the chromatin-expanding histone deacetylase inhibitor sodium butyrate to improve transcription speeds. Moreover, sodium butyrate greatly enhances levels of the wound-healing telomerase activator epiregulin after exercise by upregulating the transcription factor Sp1. Also, it is expected that testing B (Bikini Atoll... Test B!) will give better results when a low polyphenol diet is used while applying telomerase activators, because high polyphenol diets tend to generate telomerase inhibitors. Now that I have identified endogenous telomerase activators and 15 exercise-induced telomerase activators together with supplements to promote their expression, we have still more measurements to do to define optimal therapy. By February 2013, we have estimated that Product B is associated with a maximum rejuvenation rate of B = -8.218 years/year.
Of all animals, only Leach's Storm Petrel [Wikipedia, Links, Images] has telomeres which lengthen with age. The animal lives from 20 to 35 years and is black with a white mark on the tail resembling a load of salt. I get the impression that it dies of mitochondrial aging preventable in humans by using acetyl L-carnitine with alpha lipoic acid and/or pyrroloquinoline quinone (PQQ). On the other hand, the rainbow trout is a fish with high telomerase levels in all its tissues. I might add that in humans improving hTERT transcription in the nucleus improves DNA protection in mitochondria, a surprising result. hTERT transcription also improves DNA repair in the nucleus generally, although it is most famous for managing DNA repair and regrowth of telomeres.
Tables for the rejuvenation times and costs may be deduced by linear analysis.
Let the final model age tFM be given by tFM = t0 + BΔt, where
t0 is the Initial Actual Chronological Age, and B is the aging rate. Let
B = -8 or -9 years per year aging rate for rejuvenation, so that
tFM = the model age at the end of rejuvenation. In the table below we choose tFM = 25.
Solving for the rejuvenation time Δt, we find Δt = (tFM - t0)/B years.
Then the Actual Age at Final Model Age tFM is t = t0 + Δt, and we have Cost = $76.00(12)(Δt).
Note that B=1 corresponds to normal aging, B > 1 represents accelerated aging,
0 < B < 1 represents decelerated aging, and B < 0 corresponds to rejuvenation.
Rejuvenation Times to Model Age 25 from Initial Age in Years with Cost in Dollars
Initial Actual Chronological Age t010090807060504030Δt-5 Rejuvenation time, B = -5 yrs/yr 15.0.13.0.11.09.07.05.03.01.0Δt-8 Rejuvenation time, B = -8 yrs/yr9.3758.1256.8755.6854.3753.1251.8750.625Δt-9 Rejuvenation time, B = -9 yrs/yr 8.3337.2226.1115.0003.8882.7771.6660.556t = t0 + Δt-5 =
Actual Age-5 at Final Model Age 25115.0 103.0 91.0 79.0 67.0 55.0 43.0 31.0 t = t0 + Δt-8 =
Actual Age-8 at Final Model Age 25109.375 98.125 86.875 75.685 64.375 53.125 41.875 30.625 t = t0 + Δt-9 =
Actual Age-9 at Final Model Age 25108.333 97.272 86.111 75.0 63.888 52.777 41.666 30.556 Cost-5 = $76.00(12)Δt-513,680 11,856 10,032 8,208 6,384 4,560 2,736912Cost-8 = $76.00(12)Δt-88,550 7,410 6,270 5,185 3,990 2,850 1,710570Cost-9 = $76.00(12)Δt-97,597 6,586 5,573 4,560 3,5462,5331,519507
Left: Jeanne Calment, age 25. Right: Jeanne Calment, age 60.
Also see Einstein Greying, Astragalus Extract Program at 2-Year Point, and pics2013may1.html.
"...and bending down beside the glowing bars, murmer a little sadly how love fled,
and paced the mountains overhead,
and hid his face amid a cloud of stars." - When You Are Old, by William Butler Yeats.
Rejuvenation Times from 60 to Final Model Ages tFM in Years with Cost in Dollars
Final Model Age tFM5550454035302520Δt-5 Rejuvenation time, B = -5 yrs/yr 1.02.03.04.05.06.07.08.0Δt-8 Rejuvenation time, B = -8 yrs/yr0.6251.251.8752.53.1253.754.3755.0Δt-9 Rejuvenation time, B = -9 yrs/yr 0.5561.1111.6672.2222.7783.3333.8894.444t = t0 + Δt-5 =
Actual Age-5 at Final Model Age61.062.0 63.0 64.0 65.0 66.0 67.0 68.0 t = t0 + Δt-8 =
Actual Age-8 at Final Model Age60.625 61.25 61.875 62.5 63.125 63.75 64.375 65.0 t = t0 + Δt-9 =
Actual Age-9 at Final Model Age60.556 61.111 61.667 62.222 62.778 63.333 63.889 64.44 Cost-5 = $76.00(12)Δt-5912 1,824 2,736 3,648 4,5605,4726,3847,296Cost-8 = $76.00(12)Δt-8570 1,140 1,710 2,280 2,8503,4203,9904,560Cost-9 = $76.00(12)Δt-9507 1,013 1,520 2,027 2,5343,0403,5474,053
The $76.00/month cost was derived from summing the cost of 3 bottles of Solaray Astragalus Extract (3 x $8.50), 2 bottles of Herb Pharm Astagalus Extract (2 x $11.59), 1 bottle of Solaray Astragalus Root (1 x $7.25), 1/2 bottle per month of Chitosan (0.5 x $10.00), and 1/2 bottle per month of NOW FOODS IGF-1 Liposomal Spray (0.5 x $30.00). This is the basic cost of the telomerase activators used during the 1st 15 days of the two-part month-long treatment cycle. Sometimes I also rub 4 droppers of GAIA Herbs Astragalus Extract in glycerin into my scalp every day during the first 15 days. Recently in 2012, I have been taking 25 grams/day of Now astragalus root powder for the first 15 days plus GAIA astragalus root extract (Green Label, 3 bottles for 15 days), mostly rubbed into the scalp. In addition, during the first part of the cycle I take 3000 mg/day of Vitamin C, 5-10 grams of arginine per day, a Centrum multivitamin pill, 4 x 400 mg Acetyl L-Carnitine with 4 x 200 mg Alpha Lipoic Acid, 2 grams of CoQ10, 4 x Super B-vitamin pills, and 4 x 25 mg DHEA, 4 x 500 mg of NOWcolostrum, 5 x 500 mg of NOW colostrum in 1/6 cup of water for skin cream, 2 grams of ginko biloba, 3 grams of fenugreek seed before bedtime, 4 x 500 mg of black cohosh, and daily exercise. During the 2nd 15-day part of the cycle featuring telomerase inhibitors, I drop the telomerase activators and take telomerase inhibitors including green tea (3 x 60 mg ECGC), curcumin from turmeric mixed with black pepper in water, 4 x 150 mg green tea extract, 4 x 1000 mg omega-3 fish oil, 4 x 200 mg resveratrol red wine complex, and 1 x 300 mcg melatonin, cacao bean products including cocoa and chocolate, and dietary polyphenols, together with the same basic supplements used in the first part of the cycle, 3000 mg/day of Vitamin C, 5 grams of arginine per day, a Centrum multivitamin pill, 4 x 400 mg Acetyl L-Carnitine with 4 x 200 mg Alpha Lipoic Acid, 4 x Super B-vitamin pills, 3 Pomegranate softgels, 4 x 400 IU Vitamin D3, and 4 x 25 mg DHEA. Perhaps I spend $130/month (2007) to $230/month (2013) on supplements, altogether, economizing my obtaining many from discount stores. I also take 2 tablespoons of chocolate powder stirred in water, then heavily iced for antioxidant effect and for extra arginine, several times a day. I believe from toxicology studies that this is safe.
The Pomegranate and the Vitamin D3 supplements help deflect cancer, and Vitamin D3 is an anticancer telomerase inhibitor. Another alternative is Herbal Remedies Astragalus 1.25 mg astragalosides per 250 mg cap, via Nature's Way, ( Standardized 0.5% Astragalosides ), 60 VCapsules per bottle, incuding Astragalus, dried extract 250mg (root) 0.5% astragalosides, with Astragalus (root) 250mg. Four capsules yield 5 mg astragalosides plus 1 gram of astragalus membranaceus root, which improves bioavailability of astragalosides. Obviously, we need more measurements of actual results obtained with off-the-shelf astragalus root ( < 33 grams/day), astragalus extracts, and similar preparations such as TA Sciences TA-65 and RevGeneticsAstragaloside IV or cycloastragenol Astral Fruit-C to verify tables like this table for rejuvenation to effective telomeric chromosomal age 25. The pioneering research in the matter was done by Geron [Geron Patent, A', A''], TA Sciences, Telomolecular Nanotechnologies, VIDA Institute, Terraternal, RevGenetics, and other firms including Sierra Sciences. They are still trying to develop faster, more effective telomerase activators that get results quicker. Measurements of telomere growth in base pairs may be done through Repeat Diagnostics for less than $700 per pass as suggested by RevGenetics, via TA Sciences if one is enrolled in their program, via Spectracell Laboratory, via Life Length, Telome Health, or via kits and software described in our labs section. See the rejuvenation tables for my self-experiment in anti-aging therapy with astragalosides at agetransformation.html, and my video Anti-Aging Therapy with Astragalosides.
Note that decelerated aging with 0 < B < 1 corresponds to the usual program of anti-aging medicine without telomerase activation, a footdragging delay in the aging process. For instance, vitamin C or homocysteine blockers decelerate the aging process, slowing the removal of telomere base pairs from chromosome tip ends. On the other hand, the aging regimes with B < 0 correspond to rejuvenation with telomerase activation. Plotting the final model age tFM = t0 + BΔt as a function of t = t0+ Δt yields charts of aging as controlled by stepwise modification of the aging rate B(t).
Let Δt be the treatment time for a desired age transformation from a certain Chronological Age to a younger Desired Model Age. Then
(Chronological Age - Desired Model Age)/9 < Δt < (Chronological Age - Desired Model Age)/8,
where the ages are given in years and 8 and 9 years per year are the limiting absolute B-factor magnitudes for the age transformation rates.
Deaging Plot: See the Model Age spread at the 58th, 60th, 61st, 62nd, 63rd, 64th, 65th birthdays.
Based on TA Sciences 8 years per year to 9 years per year rejuvenation rate results obtained for TA-65 at 5 mg/day using the Patton Protocol. B = - 5.2 years per year was initially estimated for astragalus extract, and B = - 5.18684 shows that this was close enough for the data quality available. All model ages in the above drawing correspond to May Day, as treatment started on May 1, 2007, whereas my birthday is on May 13, 1949. After 2.5 years at about 60.5 when bone dry I seemed to be 45 to myself, whereas with oiled hair I seemed to restaurant help to be 40 at 60. This led to the B = - 5.2 years per year estimate for the astragalus extract rejuvenation rate.
By 2014, however, I note that with astragalus NK cells rejuvenate much faster than other cell types. With exercise and HGH, deaging can be more uniform, as all human cells have about the same density of GHR, the HGH receptor, in their cell membranes.
Deaging Plot Corresponding to Astragalus Extract Observations after about 2.5 years.
(From 58 to Model Age 45 at 60.5 in 2.5 years, projected to Model Age 25 at 64 in 6.33 years.)
The observed rejuvenation rate is computed at B = -5.18684 years per year, approximately B = -5.2, corresponding to the top line on the chart. At the present time, I may seem to be 45, so that at the B = -5.18684 rate I would achieve a 25 year old appearance at about 64.33 years of age in 2013. The dotted lines are for TA-65 at -8 and -9 years per year. Basic cost is about $55/month, including Chitosan, or $660/year, yielding $4,188 for 6.34 years to 25 at 64. Extra money might be spent on CoQ10, alpha lipoic acid with acetyl L-carnitine, magnesium, zinc, vitamin D, multiple vitamins, resveratrol for the off part of the cycle, and other refinements.
Anti-Aging Flight Plan (reminiscent of My World Line by George Gamow). 64.3 = August 31, 2013. Δt = 6.3 years.
Here Model_Age = Bt + Starting_Age, B=1 for normal aging, B > 1 for accelerated aging, B=0 for no aging, and B < 0 for rejuvenation, or negative aging. Here initially B= 1 for normal aging, then
B= - 5.2 years/year during the deaging phase, followed by B=0 zero aging until Madame Jean Louise Calment's 122 years, 164 days record is exceeded at 2071.9634 AD, which should be right at the Winter Solstice when shadows are shortest about Dec 22, 2071. About this time the S.Monocerotis skywalker above the Cone Nebula crosses the meridian. Note the "Cross of Years" formed by the 110 and 122.45 barriers. There are thousands of Americans over 100, of whom hardly any will get though the 110 year barrier without medicine. See the associated response of the cloud cover to the inclusion of these charts including the sign of the cross in Visionary Sky 46.5. Note that the B = 0 line in the flight plan can be implemented by using astragalus extract one month out of six, which would lead to a barely discernable jaggle on the B=0 line between 25 and 25 + 5/12 = 25.42 with a five-month rise time and a one-month fall time, since (1/12)(-5.2) = -0.43.
Phase I: For 0 < t < 57.967123 = t0, B = 1, Model Age = Bt. Ended May 1, 2007.
Phase II: For 57.967123 < t < 64.3, B = - 5.2 years/yr,
Model_Age = B(delta_t) + t0 = (B/12)N + 57.967123,
N = 1, 2, 3,...,76 months, using off-the-shelf astragalus extracts. Started May 1, 2007. See Quick Summary for details.
Phase III: For 64.3 < t < infinity, B = 0, Model Age = 25. Starts at August 31, 2013.
Note that the Flight Plan chart may be similar if the observed approximate B = -5 years per year rejuvenation rate is due to a reduction in the number of short telomere ( < 4 kbp) cells, rather than to an increase in average telomere length. That this may be true is indicated by recent research on TA-65. (Calvin B. Harley, et.al, 2010). Average telomere length growth may require hEST1A and Replication Protein A to open telomere t-loops when the length is greater than 4 kbp, or phosphorylation of tankyrase 1 with insulin from Fenugreek seeds to open telomere loops by stripping telomere loop closure protein TRF1.
On the other hand, Retinal pigment epithelial cells transfected with hTERT plasmids show telomeres lengthening at 115-255 bp per population doubling, and we find that BJ foreskin fibroblasts similarly transfected show a telomere length increase of 340-370 bp per population doubling, the same order of magnitude (400-460 bp/year telomere growth, corresponding to roughly 1 cell division per year) originally observed at TA Sciences in 2007 by Bob Waskom and Greta Blackburn. - From Andrea G. Bodnar, Michel Ouellette, Maria Frolkis, Shawn E. Holt, Choy-Pik Chiu, Gregg B. Morin, Calvin B. Harley, Jerry W. Shay, Serge Lichtsteiner, and Woodring E. Wright (1998), Extension of Life-Span by Introduction of Telomerase into Normal Human Cells, Science, vol. 279, 16 January 1998.
Estimated Rejuvenation Times = T to Target Model Age t1
from Starting Age t0 for B= -5.2 years/year
with Chronological Age After Treatment = t0 + T.
Treatment Time T(t0, t1) = (t0 - t1)/5.2 years, T(ray, target) = Treatment Time Chronological Age after Treatment = t0 + T = t0 + [(t0 - t1)/5.2] years = Final Age. Treatment Time (yr), Age after Treatment | Treatment Time (yr), Age after Treatment (yr) Start Target Duration Target Final Age | Start Target Duration Target Final Age t0 t1 T t1 t0 + T | t0 t1 T t1 t0 + T T(110, 25) = 16.346, Age(25) = 126.346; T(105, 25) = 15.385, Age(25) = 120.385, T(100, 25) = 14.423, Age(25) = 114.423; T(95, 25) = 13.462, Age(25) = 108.462, T(90, 25) = 12.500, Age(25) = 102.500; T(85, 25) = 11.538, Age(25) = 96.538, T(80, 25) = 10.577, Age(25) = 90.577; T(75, 25) = 9.615, Age(25) = 84.615, T(70, 25) = 8.654, Age(25) = 78.654; T(65, 25) = 7.692, Age(25) = 72.692, T(60, 25) = 6.731, Age(25) = 66.731; T(55, 25) = 5.769, Age(25) = 60.769, T(50, 25) = 4.808, Age(25) = 54.808; T(45, 25) = 3.846, Age(25) = 48.846, T(40, 25) = 2.885, Age(25) = 42.885; T(35, 25) = 1.923, Age(25) = 36.923, T(110, 35) = 14.423, Age(35) = 124.423; T(105, 35) = 13.462, Age(35) = 118.462, T(100, 35) = 12.500, Age(35) = 112.500; T(95, 35) = 11.538, Age(35) = 106.538, T(90, 35) = 10.577, Age(35) = 100.577; T(85, 35) = 9.615, Age(35) = 94.615, T(80, 35) = 8.654, Age(35) = 88.654; T(75, 35) = 7.692, Age(35) = 82.692, T(70, 35) = 6.731, Age(35) = 76.731; T(65, 35) = 5.769, Age(35) = 70.769, T(60, 35) = 4.808, Age(35) = 64.808; T(55, 35) = 3.805, Age(35) = 58.805, T(50, 35) = 2.885, Age(35) = 52.885; T(45, 35) = 1.923, Age(35) = 46.923, T(40, 35) = 0.962, Age(35) = 40.962.
I think perhaps these effects can be reliably mobilized for $130 x 12= $1560/year, so for each column of treatment time T,
Approximate Cost = $130 x 12 x T = $1560 x T = $9,828.00 after 6.3 years
Thus we may now have anti-aging medicine and associated technique to survive hundreds of years, although the medicine acts very slowly, like aging itself, to make us young again. Each month the expense of it satisfies
Cost/month > $130, perhaps $130 < Cost/month < $150,
depending on how thoroughly we treat every variable. Of course, still more can be spent by very careful experimenters, especially if they cautiously employ blood testing to measure their age variables and telomere lengths. By the 22nd century, the merit of telomerase activation in connection with anti-aging treatment will be obvious, but for decades to come until decisive demonstrations are readily available from many experimenters, time and expense may veil the solution. A more effective, safe enough, fast-acting anti-aging telomerase activation technique could save many lives merely by subtracting the elements of expense, long suffering, and mystery.
Fast-Acting Techniques
So far, one email correspondent has suggested that 9.5 years per year rejuvenation results have been observed with schemes like ours, and up to 460 bp/year of telomere growth has been observed with the cyclic Patton Protocol at TA Sciences using the astragalus extract TA-41 corresponding to absolute rejuvenation rates of up to 9 years per year. Subsequent observations (Calvin B. Harley, et.al, 2011) suggest that TA-65 merely closes the shortest telomere in a cell to prevent replicative senescence. Fast rejuvenation rates may be possible with adequate safety. Very high speeds have been reported using nucleoside-modified hTERT mRNA. We need to measure the telomere growth rates for the safer of the 178 various telomerase activators we have listed at varying concentrations in order to gain more penetrating insight. Also, mixtures of the telomerase activators [Index] combined for parallel activation pathways may yield superior results in the future. Better methods for improving the bioavailability of the telomerase activators used may improve the cost/benefit ratio. I note that dogs and rats can tolerate higher doses of astragalus extracts than we presently use. See Shu-Yi Yua, Hong-Tao OuYanga, Ju-Yun Yanga, Xiao-Liang Huanga, Ting Yanga, Ju-Ping Duana, Jun-Ping Chenga, Yu-Xiang Chen, Yong-Jia Yanga and Pang Qiong (2007), Subchronic toxicity studies of Radix Astragali extract in rats and dogs, Journal of Ethnopharmacology, Volume 110, Issue 2, 21 March 2007, Pages 352-355. "In conclusion, our studies clearly demonstrated that Radix Astragali Extract [Images] was safe without any distinct toxicity and side effects, the safety dosage range is 5.7–39.9 g/kg for rats and 2.85–19.95 g/kg for beagle dogs, which is equal to 70 or 35 times of that of human (0.57 g/kg, say, average BW 70 kg), respectively."
See also Astragalus Extract toxicity. Thus to rejuvenate more rapidly, it may be useful to take more astragalus extract in divided doses throughout the day. In addition, we may use chitosan (perhaps 2 grams/dose) to improve the bioavailability of astragalosides. Better measurements of astragaloside bioavailability improvements with varying doses of chitosan would be helpful, and perhaps Internet searches can help us locate more existing data. Perhaps we can use a chromatin-expanding HDAC inhibitor such as sulforaphane from broccoli sprouts, diallyl sulphide from oysters or garlic, CGK 1026, Tricostatin A, sodium butyrate [Index] or the HDAC inhibitor sodium 4-phenylbutyrate [Index], to accelerate transcription of hTERT mRNA, although this is now only solidly established for CGK 1026 and Tricostatin A. We may use a diet emphasizing low-polyphenol foods during periods in which astragalus extract is taken, in order to steer clear of telomerase inhibitors generated by high-polyphenol foods. On the other hand, short duration of the effects such telomerase inhibitors may make this unnecessary. Otherwise, we may take medicines or growth factors such as IGF-1 that phosphorylate hTERT with Akt, tending to import hTERT protein from the cytosol into the nucleus. To this we can add Fenugreek extract with < 3 grams niacinamide for NAD+ substrate synthesis, to open telomere loops with Fenugreek's 4-hydroxyisoleucine insulin stimulation, phosphorylating tankyrase 1 to remove closure protein TRF1 with poly(ADP-ribo)sylation, making telomere tips accessible to the telomerase holoenzyme, and simultaneously stimulate hTERT mRNA transcription with HIF-1 transcription factor promoted by Fenugreek's diosgenin. Note that Ginkgo Biloba also stimulates production of HIF-1. Furthermore, we may use antioxidants such as glutathione and N-acetylcysteine to retain hTERT inside the nucleus [Papers]. Parallel path activation of hTERT transcription using HGH from HGH secretagogues may also help accelerate hTERT mRNA transcription without causing unacceptable transformations due to gene mutation, gene translocation, or gene amplification that can lead to cancer.
Recovery from Cellular Senescence
I note that recovery from advanced cellular senescence may be more difficult than merely defending ourselves against the arrival of replicative senescence in the first place. Patients should probably be encouraged to start early, at the fist sign of a grey hair, to conserve their sex appeal and fortify them against atherosclerosis, cancer, and other diseases of old age [charts]. Our insight into what may be achieved with small molecule telomerase activators [Index] and supporting therapies will change as more and more very aged patients attempt rejuvenation. Ultimately, it may be useful or necessary to take steps such as inserting extra genes for antisense mRNA to oppose further accumulation of factors supporting halt of the cell cycle like P16INK4A or p21Waf1/Cip1 using Zinc Finger Nuclease technology, for instance. However, until we know whether or how fast these are consumed by ubiquitination, we will probably rely on activating hTERT mRNA transcription or inserting the hTERT gene. An extra gene for hTERT might be inserted at the 25th year, administered like an oral liposomal spray or if need be like a flu shot. However, we are approaching final victory over death from degenerative diseases associated with aging (and otherwise) as controlling factors from molecular biology come more clearly into focus and our medical bioscience techniques evolve to meet the challenges. Perhaps medicines, diet, and exercise alone will usally be adequate to achieve physical immortality without modifying the human genome.
Therapy for recovering from cellular senescence [Restoring Senescent Cells | Refs 9 | Refs 10]
Telomerase activators allow us to restore youthful patterns of gene expression prior to cellular senescence, preventing replicative senescence; we also fortify the cell against stress-induced senescence by using antioxidants to protect telomeres against ROS. Michael Fossel [Fossel, (1998), JAMA] noted that telomerase can rejuvenate senescent cells. Sierra Sciencespoints to rejuvenated skin experiments: Walter D. Funk, C.Kathy Wang, Dawne N. Shelton, Calvin B. Harley, Garrett D. Pagon, Warren K. Hoeffler (2000), Telomerase Expression Restores Dermal Integrity to in Vitro-Aged Fibroblasts in a Reconstituted Skin Model, Experimental Cell Research, 2000. "Telomerase activity not only confers replicative immortality to skin fibroblasts, but can also prevent or reverse the loss of biological function seen in senescent cell populations." However, after cells become senescent, telomerase activators are sometimes incapable of rescuing the cell, and cells experience growth arrest due to cell membrane communications failure from high caveolin levels in which the cells are not responsive to growth factors such as EGF and PDGF, and they also experience the change to the larger senescent cellular phenotype, in which they resist external stimuli and apoptosis, a survivalist response of the aging cell.
This seems to be partly due to a pinch-off of cell membrane caveolae with rising caveolin-1 levels and their subsequent internalization as detached vesicles, stopping signaling through open flask-shaped caveolae on the cell membrane in which key signaling receptors are located. Thus caveolin-1 behaves as a gatekeeper molecule for terminating signaling functions associated with endocytosis, shutting down certain membrane communications associated with sensitivity to growth factors such as EGFand PDGF as caveolin-1 levels rise, producing growth arrest. Rising caveolin-1 levels also increase p53 and p21 expression, which acts to stop the cell cycle, mediating cell cycle arrest through a p53/p21Waf1-dependent pathway. Reducing caveolin-1 status decreases p53 and p21 expression, enabling recovery from the senescent state and restart of the cell cycle by telomerase-activating growth factors. Senescent cells may be rejuvenated by sequestering FOXO transcription factors away from the nucleus to down-regulate caveolin-1 expression. This makes senescent cells once again responsive to human growth factor telomerase activators such as EGF or PDGF, cures the sluggish hyporeactivity of senescent cells, and restores the youthful cellular phenotype. After lowering caveolin-1 the cell cycle can be restarted with DNA synthesis and lowered levels of cell cycle inhibitors p53 and p21 by stimulation with EGF or PDGF, both components of colostrum. (KA Cho, SJ Ryu, JS Park, IS Jang, JS Ahn, KT Kim, and SC Park, 2003). "Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation..."
Note that folic acid (List, vitamin B9) stimulates the PI3K/AKT pathway to down-regulate caveolin-1 by sequestering FOXO factors away from the nucleus. Thus a large dose of folic acid (1 to 5 mg, avoid cramps) along with a bodybuilding workout set up to elevate EGF and PDGF with exercise and supplements such as colostrum might return a man's senescent cells rapidly to a rejuvenated state. Exercise-intensive treatment is an experiment for patients without atherosclerotic plaque, arteriosclerosis, or aortic stenosis, however.
Skin Creams for Rejuvenating Senescent Dermal Fibroblasts [Expanded Notes]
A rejuvenating skin cream might be prepared with telomerase-activating colostrum (containing EGF and PDGF) mixed with folic acid (to inhibit caveolin-1, gene CAV1) to implement restoration of senescent dermal fibroblasts. It is useful to mix it in green tea. Note that colostrum also contains VEGF, which upregulates survivin to restore senescent cells. This may be combined with treatment to simultaneously elevate cyclic AMP (with glycyrrhiza extract, exercise, or forskolin), which also downregulates caveolin-1. Magnesium and alpha lipoic acid may be added to supply required cofactors including HSP90 to support telomerase assembly. Treatment with retinol [which converts into retinoic acid (List)] might be added to this to inhibit P16INK4A, which makes recovery from the senescent state difficult. However, retinol [List] should be applied during the two-week telomerase inhibiting part of the cycle of telomerase activation followed by telomerase inhibition.
Small molecule caveolin-1 inhibitors such as N-[2-(Cyclohexy-Loxyl)-4-Nitrophenyl]-Methanesulfonamide and forskolin's cAMP may be the most effective solution, because the senescent state of the cell causes it to somewhat resist insulin stimulation and other stimulation from large molecules such as IGF-1 interacting with membrane receptors. Still, downregulating caveolin-1 with insulin or IGF-1 has at least preventative value in resisting the onset of senescence. Insulinor IGF-1 (which influences every cell in the body) can be used to downregulate FOXO and thus downregulate caveolin-1prior to the onset of senescence. Insulin-boosters include:
(1) Alpha Lipoic Acid taken 600-1000 mg after a workout can increase fat-burning and insulin-stimulated glucose uptake.
(2) Banaba Leaf Extract (taken at 32-48 mg with a postworkout shake) improves insulin sensitivity.
(3) Gymnema Sylvestre (400-500 mg with a postworkout shake < 30 min after exercise) stimulates insulin secretion.
(4) Fenugreek seed or Fenugreek Extract [Images] stimulates insulin production via
(5) 4-hydroxyisoleucine [Images] isolated from Fenugreek seeds.
Dextrose may be taken at 25-50 grams to spike insulin, along with whey protein or whey hydrolysates after a bodybuilding workout.
IGF-1 may be produced in the liver from HGH (Telomerase Activators/HGH) stemming from, say, whey protein, exercise, or HGH secretagogues such as alpha-GPC. Casein (cottage cheese) elevates IGF-1 levels, as does orally ingested colostrum[List]. Acetyl L-carnitine restores levels of IGF-1 in aging neural tissue. The growth-promoting effect of IGF-1 on brain cells is potentiated by acetyl L-carnitine plus alpha lipoic acid. Note that creatine monohydrate also stimulates the production of IGF-1, which activates the PI3K/Akt pathway, excluding FOXO from the nucleus to down-regulate caveolin-1(gene CAV1) expression and prevent cellular senescence. However, senescent cell membrane caveolae are required to communicate with IGF-1, but don't exist when caveolin-1 is too highly expressed, so that IGF-1 is more useful for preventing senescence than for reversing it. Bodybuilding exercise with creatine monohydrate suitably elevates IGF-1 to inhibit FOXO transcription factors via the IGF1/PI3K/Akt pathway, thus inhibiting caveolin-1 transcription to prevent senescence. Note that the "PI3K–Akt pathway is a major upstream signaling module leading to the phosphorylation of FOXO factors, their exclusion from the nucleus," and subsequent ubiquitination by proteasomes. - from (Dominique A Glauser and Werner Schlegel (2007)).
Treatment should be applied on a cyclic basis with telomerase inhibitors to guard against cancer. Other telomerase activators may be used on a cyclic basis with telomerase inhibitors to lengthen telomeres while striving (with colostrum and caveolin-1 inhibitors to overcome the sluggish hyporeactivity of the senescent cell membrane associated with overexpression of caveolin-1 due to FOXO transcription factor overexpression in senescence and to regain youthful cell morphology while restarting the cell cycle and rejuvenating the senescent cell. Note that caveolin-1 is up-regulated by low density lipoprotein free cholesterol, so that high free cholesterol levels could lead to cellular senescence. This may be controlled with pantethine (vitamin B5), which lowers LDL cholesterol. (van den Heuvel, Schulze, and Burgering, 2005). See also Bist A., Fielding P. E., Fielding C. J. (1997), Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol, Proc. Natl. Acad. Sci. U.S.A. 1997;94:10693–10698. This article defines the caveolin-1 promoter. Note that when senescent cells are rejuvenated by downregulation of caveolin-1, the restored cell not only recovers its youthful phenotype and sensitivity to growth factors, but also lengthens its telomeres quite substantially to a halfway point allowing typically 25 further cell divisions. Further improvements in telomere length and replicative capacity can be obtained by applying suitable telomerase activators. Unfortunately, sometimes related simple schemes can be upset by low levels of vitamin K2 resulting in aortic stenosis calcification which forbids regular hard exercise.
Caveolin-1 is downregulated by c-Myc
It turns out that c-Myc downregulates caveolin-1 expression, and that c-Myc can be upregulated by EGF, PDGF, colostrum, or more dangerously by estradiol [Ref, (Tsai L.-C., Hung M.W., et al, (1997))]. PDGF is upregulated 1.55 times by 30 minutes of exercise. Note that estradiol increases stroke incidence and should be kept within 20 to 30 pg/ml in blood samples.
References:
See Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, Park SC (2003), Senescent phenotype can be reversed by reduction of caveolin status [Papers], Journal of Biological Chemistry, 2003 Jul 25;278(30):27789-95, and also Park SC, Cho KA, Jang IS, Kim KT, Ryu SJ.(2004), Functional efficiency of the senescent cells: replace or restore? [Papers, Full Text pdf], Annals of the New York Academy of Sciences, 2004 Jun;1019:309-16. Also see A. Pieter J. van den Heuvel, Almut Schulze, and Boudewijn M. T. Burgering (2005), Direct control of caveolin-1 expression by FOXO transcription factors, Biochem J. 2005 February 1; 385(Pt 3): 795–802. See also Dominique A Glauser and Werner Schlegel (2007), The emerging role of FOXO transcription factors in pancreatic b cells [Papers], Journal of Endocrinology (2007) 193, 195–207. See also Brain P. Ceresa and Sandra L. Schmid (2000), Regulation of signal transduction by endocytosis [Papers], Current Opinion in Cell Biology 2000, 12:204–210, which explains the role of caveolae in signal transduction.
Survivin and Cellular Senescence Recovery
Note that cellular senescence may also be recovered from by upregulating survivin. Survivin is upregulated by VEGF and bFGF (FGF2), both of which are contained in colostrum [List], which coincidentally contains insulin and improves expression of IGF-1 when taken orally. Note that VEGF is upregulated 1.36 times by 30 minutes of exercise. "Senescence is a reversible process controlled by survivin: by overexpressing survivin in senescent cells, we are able to decrease senescent markers and increase cell proliferation." (Caterina A. M. La Porta, Stefano Zapperi, James P. Sethna (2012), Senescent Cells in Growing Tumors: Population Dynamics and Cancer Stem Cells, January 2012 Issue of PLoS Computational Biology). Survivin should be upregulated on a cycled basis with telomerase inhibitors to guard against cancer.
N-[2-(Cyclohexy-Loxyl)-4-Nitrophenyl]-Methanesulfonamide (NS-398) for Recovering from Senescence
A selective COX-2 inhibitor inhibits senescence in one case, and decreases expression of caveolin-1 in the cell membranes of senescent cells, enabling recovery from cellular senescence when the cell is stimulated with telomerase-activating growth factors such as EGF or PDGF. The use of selective Cox-2 inhibitor N-[2-(Cyclohexy-Loxyl)-4-Nitrophenyl]-Methanesulfonamide [NS-398, 3D, Toxicity] has been patented for this application by S.C.Park and J.A.Han (2008-2012). This substance is believed to transcriptionally inhibit caveolin-1 expression. Side effects are still in question, although the selective COX-2 inhibitors lack the side effects of conventional NSAIDS. See Restoring Senescent Cells, Therapy for Recovering from Cellular Senescence, and small molecule caveolin-1 inhibitors [Papers, Patents].
Forskolin down-regulates levels of caveolin-1 via cyclic AMP to Rejuvenate Senescent Cells
Forskolin is the active ingredient in coleus forskohlii [Images, Video, Papers, Books], which activates the enzyme adenylate cyclase, which converts ATP to cyclic adenosine monoposphate (cAMP). Creatine monohydrate produces more ATP for forskolin-activated adenyl cyclase to convert to cAMP. Epinephrine also acts to stimulate adenyl cyclase to produce cAMPfrom ATP; see epinephrine supplements and epinephrine boosters, such as coffee caffeine, which inhibits cAMP phosphodiesterase, the enzyme that metabolizes cyclic AMP. Two amino acids phenylalanine and tyrosine, sold as supplements, are converted into dopamine. Dopamine, in turn, is converted into norepinephrine, and then epinephrine, which produces more cAMP from ATP. (after Ray Sahelian on Neurotransmitters.) Cyclic AMP (a second messenger) acts by activating protein kinase A (cAMP-dependent protein kinase), which can phosphorylate specific proteins that bind to promoter regions of DNA, causing increased expression of specific genes, or phosphorylate proteins that may act directly on a cell's ion channels, or phosphorylate proteins that may become activated or inhibited enzymes. "It is known that forskolinvia cAMP can down-regulate mRNA levels (of caveolin-1) in a dose-dependent manner.". - after A. Pieter J. van den Heuvel, Almut Schulze, and Boudewijn M. T. Burgering (2005), Direct control of caveolin-1 expression by FOXO transcription factors [Papers, Patents, Books] Biochem J. 2005 February 1; 385(Pt 3): 795–802. The "senescent phenotype, however, can be reversed by reducing the expression levels of caveolin-1, suggesting that either the senescent state is a reversible phenotype or that these cells are actually quiescent, rather than senescent.". See upregulating cAMP and supplements for upregulating cAMP. Schizandra berry is known to increase cyclic AMP in the liver, and licorice (Glycyrrhiza extract) also promotes cyclic AMP and inhibits cAMP phosphodiesterase, which degrades cAMP. "The effect of one bout of intense swimming caused significant increases in the cyclic AMP content of fast-twitch white skeletal muscle, liver, and heart. Further investigation of the exercise-induced increase in myocardial cyclic AMP indicates that the nucleotide content remained elevated long after (24 h) termination of exercise. This increase in cyclic AMP was time dependent, with the level increasing gradually throughout the work bout.
The increase in cardiac cyclic AMP seemed to be independent of work intensity, provided that work time was of sufficient duration (greater than or equal to 30 min)." - After Palmer WK (1988), Effect of exercise on cardiac cyclic AMP, Med Sci Sports Exerc 1988 Dec;20(6):525-30. Note that 2 ligands of the EGF receptor, epiregulin and amphiregulin, are elevated by exercise, as is PDGF, so that exercise reduces caveolin-1 levels with cyclic AMP, restoring senescent cell communications through the cell membrane, then restores senescent cells with longer telomeres via EGF family growth factors (See also EGF), PDGF, and other telomerase activators from exercise. Negative effects of high cAMP may include high body heat associated with thermogenesis and weight loss, estrogenic side-effects from stimulation of aromatase activity, and depression if dopamine levels are not kept up. Again, forskolin therapy may be combined with cyclic telomerase activation to induce telomere growth to restore and rejuvenate senescent cells. See also Yamamoto M., Okumura S., Oka N., Schwencke C., Ishikawa Y. (1999), Downregulation of caveolin expression by cAMP signal, Life Sci. 1999;64:1349–1357. Note, however, that in some cell types cAMP induces cell cycle arrest. (van den Heuvel, Schulze, and Burgering, 2005). For instance, forskolin inhibits colon cancer cell growth and survival (Wikipedia/Forskolin), and forskolin may also suppress leukemia progression (LEF, Nov 15, 2005).
Experimental siRNA restoration of Senescent Cells
These experiments down-regulated caveolin-1 using siRNA, targeting 5 regions of caveolin-1 mRNA, using 21-nucleotide siRNAs obtained from Dharmacon Research (Lafayette, Co). Caveolin-1 was also downregulated with caveolin-1 antisense oligonucleotide (TTTGCCCCCAGACAT) transfected with Lipofectamine. After lowering caveolin-1 the cell cycle can be restarted with DNA synthesis and lowered levels of p53 and p21 by stimulation with EGF (a component of colostrum). (KA Cho, SJ Ryu, JS Park, IS Jang, JS Ahn, KT Kim, and SC Park, 2003). "Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation..." Small interfering RNA oligonucleotides directed against caveolin-1 have included siRNA described in (van den Heuvel, Schulze, and Burgering, 2005).
Arginylglycylaspartic acid for Senescent Cell Morphology Restoration
Most recently treatment with the tripeptide Arg-Gly-Asp (RGD, or Arginylglycylaspartic acid [Links, Images, Papers, Patents, Books; 3D; Toxicity]) has transformed senescent human fibroblasts back to their "young-like phenotype" morphology in 5 days. H. Choi, J.H. Rhim, S.J. Lee, K.A. Cho, S.C. Park (2012), Induction of morphological restoration of senescent, Sens Foundation, 2012.
SIRT1 Deacetylates and activates Ku when Young Extracellular Matrix rejuvenates Senescent Cells
Young extracellular matrix can rejuvenate senescent fibroblasts. During the process, SIRT1 influences DNA repair and deacetylates and activates the chromosomal fusion protection and telomere protection heterodimer Ku composed of Ku70and Ku80 (Ku86). Thus SIRT1-boosters like caloric restriction and resveratrol seem quite promising in connection with recovery from the senescent state of the cell. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix, Aging Cell, 2011 Feb;10(1):148-57. See the related patent by Sang Chul Park, Kyung A Cho, Moon Kyung Ha and Hae Ri Choi, (2011 & 2009), Senescence Control Composition Containing Extracellular Matrix Components[Links, Papers], US20110150899.pdf (2011).
Tocotrienol-rich fraction can reverse senescence in human diploid fibroblasts
Gamma tocotrienol [List] reduces expression of collagenase from senescent fibroblasts, protecting the extracellular matrix, and has favorable impact on gene expression in both senescent and normal fibroblasts, tending to oppose aging. Tocotrienol-rich fraction containing alpha-tocopherol and tocotrienols alpha, beta, gamma, and delta from rice bran, palm oil, oat, or barley, tends to produce telomerase activity in senescent fibroblasts, lengthens telomeres, restores fibroblast morphology, and restarts the cell cycle. [Images/Tocotrienol-rich fraction supplements with alpha-tocopherol from palm oil; Images/Tocotrienol-rich fraction skin cream with alpha-tocopherol from palm oil]. See Suzana Makpol, Azalina Zainuddin, Kien Hui Chua, Yasmin Anum Mohd Yusof, and Wan Zurinah Wan Ngah (2012), Gamma-tocotrienol modulation of senescence-associated gene expression prevents cellular aging in human diploid fibroblasts [NCBI DOC], Clinics (Sao Paulo) 2012 February; 67(2): 135–143, and also Makpol S, Durani LW, Chua KH, Mohd Yusof YA, Ngah WZ (2011), Tocotrienol-rich fraction prevents cell cycle arrest and elongates telomere length in senescent human diploid fibroblasts, [NCBI DOC], Journal of Biomedicine and Biotechnology 2011 Mar 30. Gamma tocotrienol has increased animal lifetimes by up to 18%.
Geron
Midnight Blue: Astragalus Extract to the Scalp Darkens Hair in About 12 Months
Jim Green - Left: Jan 24, 2010, Age: 60.7. Right: Jan 18, 2011, Age: 61.68, Math Model Age: 38.71.
After about a year of astragalosides to the scalp, hair color improved as telomere cell DNA was repaired, activating quiescent hair follicle stem cells in the hair follicle bulge region, initiating cycles of proliferation and hair synthesis.Human dermal fibroblasts can divide about 50 times in culture, as Hayflick & Moorhead discovered in 1961. But with telomerase enzyme enough, the cells become immortal, and have divided hundreds of times in culture. I took TerraternalAstragaloside IV 100 mg/day during the activation part of the cycle during the last few months. GAIA Herbs astragalus root extract in glycerin at 1 mg astragalosides per 30 drops was used for the scalp. HGH to promote hTERT mRNA transcription was boosted by 1200 mg/day Alpha GPC plus exercise. I decided it would be best to include direct topical application, as Artandi Labs at Stanford University reported positive hair restoration results on experimental animals with direct application of astragalosides to hairy skin. "Here we show that conditional transgenic induction of TERT in mouse skin epithelium causes a rapid transition from telogen (the resting phase of the hair follicle cycle) to anagen (the active phase), thereby facilitating robust hair growth. TERT overexpression promotes this developmental transition by causing proliferation of quiescent, multipotent stem cells in the hair follicle bulge region." - after Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE (2005), Conditional telomerase induction causes proliferation of hair follicle stem cells, Nature, 2005 Aug 18;436(7053):1048-52. Most recently (August 2011) I have tried using an ethanolic extract of Fenugreek seed as a telomerase activator [List] on my scalp, which is relatively inexpensive. Colostrumsolution was also under test for treating hair greying. Nothing worked well except application of green flag GAIA Herbs astragalus extract in alcohol or glycerin to the scalp, which takes about a year to modify hair color from the roots out. Further Remarks.
Anti-Aging Medicine: Life Extension in Review || 1 | 2 | 3 | 4 || Index Change Log
Longevity 1 | 2 | 3 | 4 | Bibliography | Labs 1 | 2 | 3 | Foots | Refs | Sup Notes 1 | 2 | 3a | 3b1 | 3b2 | 3b4 | 3b5 |
3b6 | 4 | 5 | Vendors | Change Log | Index Change Log | Age Transformation | Special: Restoring Senescent Cells
Topics | Am | An | B | Ca | Cb | D | E | F | G | Hs | Ht | I | J | K | L | Mc | Md | N | O | Pq | Pr | Q | R | Sl | Sm |
Te | Tf | U | V | W | X | Y | Z | Topics | More Topics | Health Topics || Home | Dental
JenAge/Blogs | Anti-Aging Firewalls | Fighting Aging | Biology of Aging/Blogs | MethusFoundationBlog | Biosingularity | @aging Blog | LifeExtension Blog | William Faloon | Dr. Jerry Shay | Dr. Woodring E. Wright
Videos : Immortality & Anti-Aging Medicine & Telomeres & Telomerase & Longevity & Medicine & Stem Cells & DNA Science & Gerontology & DNA Damage || Dr. William Andrews | Dr. Titia de Lange
& DNA Repair & Telomeres & Telomerase & E. Blackburn & Cellular Senescence & Replicative Senescence & M.Fossel & Nutraceuticals (HPlan) & Neuro & Aubrey de Grey: SENS Therapies || SENS | Henry Stewart Talks
& Cells & Skin & Detox & Heart & Cancer & Stroke & Neck Rejuvenation [Exercises] & Facial Exercises || Senior Journal || Solaray | Herbal Remedies | QC | Ray | AC-11 | Barron/End of Old Age & Dr. Carol Greider
Mechanisms of Aging | INNOVITA | SENS | Fight Aging | Methusalah Foundation | JenAge || Essential Evidence Plus | MIT | Molecular Biology of Aging Refs 2 | Hayflick Limit Papers | Longevity Genes
PubChem | PubMed | Labome |[ Wikipedia | Wikigenes | iHOP | SA Biosciences ]| Geron/Telomerase A || Cycloastragenol+: TA Sciences | Iron-Dragon | King Tiger | Genescient | Dr. Al Sears
AGE inhibitors | Alzheimers | Anti-Inflammatory | Cardiology | Antioxidants || Anticancer || Telomerase Inhibitors || Telomerase Activators | Sierra Sciences | IsAGenix | Product B | Terraternal | RevGenetics
Search: Journals | Basic Calc | Sci Calc | PubMed | NIH | MedLine | WebMD | HealthLine | Access Medicine | Wiki/Medicine | Medical Diagnosis | Truth In Aging || Clinical Trials | BioMedSearch | Discovery Medicine/Telomere
Google |[ Patents | Patent Lens ][ Books | LibCong | Amazon | Powells ][ Google Scholar | Wikipedia ][MedLib | LibWeb] | Merriam-Webster | Cope | HUGO | U.S. National Library of Medicine | Discovery Medicine
| LEF| Aging Cell| Rejuvenation Research| IAAS| A4M| ARC| ImmInst| SWL | Groups/LEF | Guide | MF | Immortal Humans
(6) The insulin utilization theory [Index/Insulin, Links/Insulin and Aging, Images, Papers, Patents, Books; Books/insulin_resistance, Amazon, LifeExtension/insulin_and_aging,
LifeExtension/insulin_utilization; Index/Diabetes].
Chromium picolinate can be used to metabolize extra insulin.
Treatment with chromium picolinate [Images] has been shown to increase the life span of mice 15%. There are some reports that chromium picolinate may be harmful to DNA [Links], and is primarily useful in losing weight. Perhaps some other source of the chromium ion should be used to impact insulin metabolism and help with the weight loss factor related to the caloric restriction theory (3). See also insulin and insulin resistance, Dr. Lam's Insulin and Aging, and Life Extension Magazine on metabolic syndrome and insulin resistance. It is desirable to avoid or deemphasize sugary foods and consume foods with a low glycemic index (*, Links) that cause glucose to rise more slowly to avoid obesity, metabolic syndrome and insulin resistance problems.
Glucose has a glycemic index of 100. Metformin makes cells less insulin resistant, and testosterone from bodybuilding, injections, or stick-on patchesprevents and treats insulin resistance in men. "Hyperinsulinemia (excess insulin in the blood) has been implicated as a major risk factor" in numerous age-related diseases, such as Alzheimer's disease, heart attack, and stroke. (See Fantastic Voyageby Ray Kurzweil and Terry Grossman [Clinic, Ray & Terry's]). Alpha lipoic acid has been shown to improve insulin-stimulated glucose disposal at 100-300 mg twice a day, and drugs used to treat metabolic syndrome include chromium 200 mcg x 2 or 3/day, alpha lipoic acid, EPA/DHA (fish oil) 1000 mg/day, co-enzyme Q 60-100mg x 2, Carnosine 500 mg x 2, magnesium 200-400 mg/day, CLA 500-1,500 mg x 2/day, 500-1,500 mg x 2, L-carnitine 600 mg x 2 or 3 per day, vitamin E400-800 IU/day, vitamin C 2000 mg/day, biotin, arginine 3 grams x 3, glutamine 500-1000 mg, DHEA 25 mg x 2, and N-acetyl-cysteine (NAC) 500 mg x 2/day. Otherwise, the glycemic index of food may be effectively lowered by dietary fiber or by Precose, and Glyset prescription medicines, which slow down absorption of carbohydrates from the digestive tract and by methods from caloric restriction.
The more sugar insulin transports into cells [Books], the more vulnerable the cell to glycation reactions [Papers, Patents, Books] producing carbonylated proteins [Images, Papers, Patents, Books; Patent] that may clog up proteasomes (14). Glycation reactions with DNA [Papers, Patents, Books] may also take place. This is probably the reason that genes like daf-16 [Links, Images, Papers, Patents, Books] in C. Elegans involved in insulin management are deemed "longevity genes" [Links, Images, Papers, Patents, Books; 0.2].
Note that for bodybuilding purposes, it is desirable to elevate insulin levels after an evening workout before bedtime to sweep nutrients into cells and restore muscle glycogen with insulin-boosting drugs such as Gymnema Sylvestre or Fenugreek extract, using a two-week on/off cycle to prevent breast enhancement. Elevating insulin makes one tired as blood glucose goes down, and eventually hungry, too.
(7) The advanced Hayflick cell division limit theory based on telomeres [Refs1b.7, Refs2.7, Fortune/Youth Pills, Books, Amazon, LifeExtension/telomere_therapy, Mechanisms of Aging/telomeres, Innovita, End Replication Problem, History of Telomeres and Telomerase, History of Telomerase Activators, Hayflick, His Limit, and Cellular Aging by Jerry Shay and Woodring E.Wright, telomeres & mortality, Ben Best/Telomeres and Aging; Mouse, the Unbeliever: SenescenceInfo/Telomeres & Telomerase, and Geraldine Aubert and Peter M. Lansdorp, Telomeres and Aging, Physiol. Rev.88: 557-579, 2008.] - Revolutionary advanced methods based on telomerase technology or alternate technology for allowing mitotic cell division to continue indefinitely [Geron] following gene therapy or alternative treatment to re-extend telomeres with telomerase activators [Index, List, 81s]. In vertebrates, telomeres at chromosome tip-ends [Books] consist of tandem arrays of minisatellite [5'-GGTTAG-3']n repeats from 4,000 to 15,000 bp long. These are often described as T2AG3repeats in the literature (5'-TTAGGG-3') (E. Blackburn, 2001). I have specified the sequence as [5'-GGTTAG-3']n (or G2T2AG), following the template region of the hTR RNA molecule (W Klapper et al, 2001, in Mark P. Mattson, 2001). "The template region of TERC is 3'-CAAUCCCAAUC-5'. This way, telomerase can bind the first few nucleotides of the template to the last telomere sequence on the chromosome, add a new telomere repeat (5'-GGTTAG-3') sequence, let go, realign the new 3'-end of telomere to the template, and repeat the process. " - Wikipedia/Telomerase.
TELOMERASE TERC Template RNA (hTR template RNA) 3'-CAAUCCCAAUC-5' ...GGTTAGGGTTAGGGTTAGGGTTAG-3' New Telomere Hex Repeat DNA
The first 5 nucleotides of the RNA TERC (hTR) template bind to the last 5 GTTAG nucleotides (shown in red) of the last hex repeat. After formation of the new DNA hex repeat GGTTAG the TERC (hTR) template steps right 6 nucleotides to align the next new hex repeat. "Normal human cells stably expressing transfected telomerase can maintain the length of their telomeres, and exceed their maximum lifespan by more than fivefold." Furthermore, normal cells immortalized with telomerase do not become cancerous [article], although 85% of known cancer cells express telomerase to enable them to keep dividing. In fact, it is believed that cellular telomeric crisis [Index/Telomere Fusion, Links, Images, Papers, Patents, Books, Amazon] due to shortening of cellular telomeres causes carcinomas [in breast cancer, Books]. Cytological observations of broken chromosome ends [Links, Images, Papers, Patents, Books, Amazon] by McClintock in maize in the 1940s [Books] led to the suggestion that telomeres functioned to prevent chromosomal end-point fusions, which have been recently shown to sometimes lead to cancer. Sperm cells and oocytes (germ cells) manifest high telomerase levels without showing cancer problems. When telomerase is applied exogenously to mitotic human cell cultures, they become immortal. Telomerase therapy even works to improve life span in long-telomere animals such as mice [Ref, 2012].
The Structure and Location of hTERT and hTR telomerase components
The hTERT component
I note that the hTERT protein catalytic component of telomerase is generated by the hTERT gene [Index, Links, Images, Video, Papers, Patents, Books, Amazon], consisting of 16 exons and 15 introns spanning ~37 kb of genomic sequence (Wick et al., 1999) near the distal end of chromosome 5p at 5p15.33, probably the most distal gene on the chromosome, which contains 609 genes. The half-life of the associated molecule (hTERT mRNA transcript half-life (1-3 hours), hTERT protein half-life (4 weeks), half-life of the active telomerase complex (24 hours)) is up to 4 weeks. (See Xiaoming Yi, Jerry W. Shay, and Woodring E. Wright, Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells, Nucleic Acids Res, 2001 December 1; 29(23): 4818–4825). The hTERT protein, the hTERT catalytic component of telomerase, includes 1132 amino acids with a total molecular mass of 127 kDa. The information for the transcribed part of hTERT mRNA is contained in 1132 x 3 nucleotides + 3 stop nt + 3 start nt = 3402 nt, and together with the 5' cap, the 5' UTR, the 3' UTR and the poly(A) tail of mRNA structure, hTERT mRNA is contained in 4015 nt. One microgram of 20 nt of single-stranded RNA yields 9.17 x 1013 molecules, so 1 microgram of 4015 nt of hTERT mRNA yields (20/4015) x 9.17 x 1013 = 457 x 109 hTERT mRNA molecules. For purposes of viral or plasmid transfection, it may be useful to prepare condensed hTERT cDNA with various promoters.
Adding the 16 exon lengths of hTERT together, I find 3396 nt length, 3.396 Kb. The CMV promoter length is 508 nt, so that hTERT cDNA with a CMV promoter and without introns should come to 3904 nt or 3.904 Kb. Using the canonical 181-bp hTERT core promoter 19 bp upstream of the first nucleotide in the cDNA sequence instead would yield hTERT cDNA + core promoter = 3396+181+19 nt = 3596 nt = 3.596 Kb. The hTERT protein structure is highly similar to retrotransposons and includes the motifs 1, 2, A, B', C, D, and Echaracteristic of the active sites of retrotransposons. In addition, telomerases contain the hTERT T-motif and show a larger distance between the A and B' motifs than is found in retrotransposons. Our problem of telomerase activation to repair shortened old telomeres and allow more cell divisions is primarily dependent on our ability to supply, use, or activate transcription factors that interact with the hTERT promoter to produce hTERT mRNA used to produce the catalytic component of telomerase. This process may be accelerated by HDAC inhibitors that act to expand chromatin by acetylating DNA, promoting transcription and counteracting gene silencing. Otherwise, telomerase activation is typically produced by phosphorylating cytoplasmic hTERT with kinases (such as AKT1 kinase) for import into the nucleus. Fast telomere extension using purified nucleoside-modified hTERT mRNA in liposomes was announced in 2013. This method extends telomeres in six days by an amount by which telomeres shorten over approximately 15 years of normal human aging.
The hTR component
The hTR sequence for the RNA polymerase II-transcribed RNA part of telomerase is located on chromosome 3 (1,436 genes) at 3q26.3, and the associated hTR mRNA exhibits a half-life of about 5 days. Check the length of the hTR mRNA transcript. All vertebrate hTR RNA uses the template yielding the hex nucleotide DNA extension sequence [5'-GGTTAG-3']n, includes up to 35 RNA sequences, and features 4 conserved structural domains: the CR/C5 region, the psuedo-knot, the H/ACA box (for localization of telomerase hTR RNA to the nucleolus), and the CR7 region (W Klapper, R Parwaresch and G Krupp, 2001). It seems likely that the H/ACA domain of hTR RNA binds to H/ACA proteins formed by snoRNAs such as dyskerin, which is found in the nucleolar telomerase complex.
In adult tissues, hTR is present and most highly expressed in the spermatocytes and Sertoli cells of the testis, moderate expression is observed in germinal center lymphoid follicles, and weak expression is present in regenerative cellular epithelia, but is not seen in the nervous system and mesenchymal derived tissues including connective tissue, bone, cartilage, and the circulatory system and lymphatic system cells. Treatment with mesenchymal stem cells is one method of handling their eventual failure due to low hTR expression in regenerative medicine.
See Nedime Serakinci, Stacey F Hoare, Moustapha Kassem, Stuart P Atkinson & W Nicol Keith (2006), Telomerase promoter reprogramming and interaction with general transcription factors in the human mesenchymal stem cell, Regenerative Medicine, Jan 2006, vol.1, No.1, pp 125-131 (Summary in Future Medicine). "It is shown that repression of hTERT expression in hMSCs (human Mesenchymal Stem Cells) is due to promoter-specific histone hypoacetylation coupled with low Pol II and TFIIB trafficking. This repression is overcome by treatment with Trichostatin A (TSA), an HDAC inhibitor, concomitant with increases in promoter-specific histone acetylation and increases in Pol II and TFIIB tracking. hTR expression is also increased in TSA-treated hMSCs, concomitant with changes in Pol II and TFIIB dynamics." It is probably true that hyperacetylation with sodium butyrate or sodium butyrate chicken feed would produce equivilant results.
Garlic improves the Hayflick limit of dermal fibroblasts, probably due to the HDAC inhibitor properties of diallyl disulfide and allyl mercaptan metabolites of allicin from garlic, which expand chromatin for better transcription of hTR and hTERT mRNA. HDAC inhibitor properties have recently been discovered for L-carnitine, so that this common nutraceutical (obtainable from methionine from fish in the presence of lysine and vitamin C) might also be used to overcome poor expression of hTR and hTERT in mesenchymal-derived tissues via hyperacetylation of chromatin. See hTR and the hTR Promoter. Several "putative" binding sites for the glucocorticoid, progesterone [hTERT_promoter], and androgen steroid hormones have been found in the 5' flanking region of the hTR gene and may also be present in the hTERT gene (C.J. Cairney and W.N. Keith, 2007, 2008).
Expression of hTR in adult tissues is predominantly limited to dividing cells, although certain differentiated postmitotic cells express hTR. [Yashima K, Wright WE, Shay JW, et al., 1998, Papers/hTR activation, Papers/small molecule hTR activators, J. Zhao, et al, 2003, Links/telomerase RNA gene activation, Links/hTR plasmids, Links/hTR transfection]. The hTR or hTERC promotor is silenced via methylation [Index, Links, Papers]. Sp1 (binding at GC boxes) and HIF-1 are positive regulators of hTR transcription (C.J. Cairney and W.N. Keith, 2007). Note HIF-1 can be upregulated by hypoxia from exercise, ginkgo biloba, or by fenugreek seed, while Sp1 is upregulated by sodium butyrate [Images] and down-regulated by insulin deprivation. Perhaps these can be applied to solve the special problems involving mesenchymal-derived tissues. Note, however, that sodium butyrate induces growth arrest (in cancer cells) by inhibiting DNA synthesis, arresting actively proliferating cells in G1 to induce differentiation. This suggests that perhaps it should not be used continuously. Sodium butyrate supplements are sometimes shown with a rooster-waggle neck picture, probably to suggest that they may be good for neck wattles composed of old connective tissue derived from mesenchymal cells by boosting the expression of hTR there via elevated Sp1. Increased Sp1 binding to promoter regions is seen in cells that overexpress the endogenous antioxidants Cu/Zn-SOD (SOD1) and/or catalase [Index], and curcumin has been observed to increase Sp1 binding. Recently it has become clear that hTERT and hTR are better expressed in mesenchymal-derived tissues when HDAC inhibitors such as sodium butyrate or L-carnitine are used to expand chromatin for transcription.
Telomerase Fractionation
Fractionation of the telomerase enzyme from nuclear extracts with a glycol gradient yields a size of about 1000 kDa for the native enzyme and about 550 kDa for affinity purified telomerase, the difference being due to loosely associated factors lost during purification (W.Klapper et al 2001). A single hTERT molecule accounts for just 127 kDa. In addition, we have the hTR RNA, dyskerin, the shelterin or telosome proteins, and associated mammalian telomere protein factors. Two molecules of dyskerin, two molecules of hTERT protein catalytic component of telomerase, and 2 molecules of telomerase RNA are found in telomerase reverse transcriptase, according to recent research. This may be two molecules of telomerase bound at the RNA template, however. See Scott B. Cohen, Mark E. Graham, George O. Lovrecz, Nicolai Bache, Phillip J. Robinson, Roger R. Reddel, (2007), Protein Composition of Catalytically Active Human Telomerase from Immortal Cells, Science, 30 March 2007: Vol. 315. no. 5820, pp. 1850 - 1853. "Dyskerin is a component of a small nucleolar ribonucleoprotein(snoRNP) that is highly conserved throughout evolution.... findings indicate that dyskerin plays a role in telomeremaintenance by stabilizing TERC." - - H.-Y. Du et al. Telomerase Mutations and Premature Aging in Humans.
Molecular Densities of Telomerase
The abundance of telomerase molecules is low in cells, featuring for instance 3000-5000 copies of hTR and 30 copies of hTERT protein per cell in in vitro cultures of growing tumor cells that are the richest source of telomerase. (W. Klepper, et al. 2001). Of course, we may also inquire concerning the abundances of telomerase, hTR RNA, hTERT protein, and dyskerin molecules in sperm cells, dermal fibroblasts, hemopoietic stem cells, immortalized cells, and other types of cells under varying conditions at as a function of age.
Telomere Lengths
The lengths of different chromosomes correlate directly with telomere length [Images], and the telomeres are shorter the closer they are to the centromere [Images]; p-arm telomeres are shorter than q-arm telomeres [Images]. The lengths of telomeres may vary between alleles at the same telomere in a diploid cell. The shortest telomere in human cells is at 17p[Links, Images, Papers, Books, Wikipedia/Chromosome 17, Ornl, Genes & Disease]. The hTERT catalytic component of telomerase available is the primary limiting factor in telomerase expression, and transcriptional regulation of hTERT gene expression rather than of hTR for the RNA component of telomerase is central in replicative senescence and immortalization. Replicative senescence is pro-inflammatory and pro-carcinogenic, although it limits uncontrolled tumor growth. Inhibiting telomerase causes cancer cells to self-destruct via apoptosis unless telomerase control is via the ALT mechanism, so that telomerase inhibitors are often useful in squelching cancers. Mammalian telomeres of maximum reported length 30,000 base pairs shorten by 50-200 base pairs with each S phase of the cell cycle, starting with typically 15,000 to 20,000 base pairs in the germ line with typically 7,000-10,000 base pairs in human adults, and counting down to 4,000-7000 base pairs in senescent cells at the M1 senescent cell checkpoint preceding a conditionally triggered countdown to the M2 crisis state before apoptosis, end-point fusions, and other phenomena associated with cell death or cancer set in.
Human germline cell telomeres are maintained at about 15 kbp. Telomeric t-loops [Index] thousands of base pairs long are uncapped to reach the M1 senescence checkpoint, when the length of telomere has shrunk so that the loop tries to close at a spot where non-canonical subtelomeric repeats are encountered differing from the tandem telomeric hexanucleotide repeats [5'-GGTTAG-3']n, so that the TRF1 t-loop formation protein [Index] closing the t-loop cannot find a proper binding site. In experiments, fibroblasts [Images] with telomeres shortening up to 200 base pairs per cell division were found to loose only 5-20 bp per cell division when the cell was equipped with the most effective antioxidant defense. (This might motivate using lipid-soluble antioxidants along with water-soluble oxidants like vitamin C, or antioxidants soluble in lipids and water like alpha lipoic acid, the recommended alternative.) Cells of the healthy colon mucosa typically loose 44 base pairs per cell division. On the other hand, patients 77.5 years old were shown to have white blood cells that lost 71 telomeric base pairs per year [Nazmul Huda, et.al, 2007], perhaps showing some old-age acceleration in telomere erosion. The same paper showed that telomere lengths nearly matched between elderly twins. In one 2003 study, patients over 60 years old with shorter telomeres in blood DNA had poorer survival than patients with longer telomeres, attributable in part to a 3·18-fold higher mortality rate from heart disease and an 8·54-fold higher mortality rate from infectious disease. (Richard M. Cawthon, Ken R. Smith, Elizabeth O'Brien, Anna Sivatchenko, Richard A. Kerber, 2003). Other studies have pictured more precisely how mortality varies with telomere length in blood leukocytes, and shown how cancer incidence and mortalityvary as a function of telomere length.
Telomerase and Aging in Non-Mitotic Cells and Mitochondria [Links, Images, Papers, Patents, Books].
Note that replicative senescence does not apply in quite the same way to non-mitotic cells such as muscle cells [Links], muscle satellite cells [Links], cardiac myocytes [Links], and most nerve cells, which appear to age via other mechanismsinvolving the cell membrane, cell membrane permeability changes from hydroxyl radical reactions, membrane damage from ROS, accelerated ROS from AGEs, and internal waste accumulation. (Sometimes such cells are renewed by stem cells from bone marrow, as in the the case brain microglial cells [77s], so that the telomeric state of mitotic stem cells may be behind the continued health of non-mitotic tissue.) However, Geron claims that in vitro cultures of non-mitotic nerve and muscle cells as well as mitotic cells are functionally benefited by telomerase activation, listing 20 cellular types [81s]. It was later discovered that the hTERT protein defends mitochondrial DNA and improves DNA repair in nuclear DNA. "In many cases, introduction of active telomerase also increases the capacity of cells to withstand stress due to high or low levels of oxygen, toxic molecules, or abnormal growth conditions." Experiments in 2007 have succeeded in immortalizing muscle cells in in vitro culture using a combination of hTERT and cyclin-dependent kinase 4.
See cyclin-dependent kinase 4 [Papers, Patents, Books], which uses ATP to phosphorylate proteins and is subject to inhibition by p16INK4A and other INK4 family members [Ref]. When grown in in vitro cultures, human (73-78 year lifespan) embryonic cells divide about 50 times before reaching their telomere-imposed cell division limit, mouse (3 year life span) cells divide 15 times, and the cells of Galapagos tortoises (175 year life span) divide 90 times. Cells from human adults, on the other hand, were observed to typically divide 20 times, or 50 times from the embryonic stage of development. Telomerase activation by small molecule telomerase activators [81s/TA, List, Index] such as astragalus root extract, black cohosh, fenugreek extract, testosterone, colostrum growth factors, Nitric Oxide (NO) from arginine, HGH from alpha-GPC with exercise, IGF-1, or CGK1026, however, can confer an immortal phenotype on cells by bypassing the usual cellular senescence pathway, and can restore a youthful phenotype to aging cells with short telomeres.
Carnosine and Antioxidant Telomere Defense
"In 1999, Australian researchers confirmed that carnosine increases the longevity of human fibroblast cells in the laboratory. Carnosine extended the Hayflick limit (the maximum number of times a cell can divide), from a "normal" 50 by up to an additional 10 times!" [69] See Life Extension on Carnosine and Cellular Senescence and The Barron Report. Note that von Zglinicki et al. showed that cells grown in a high oxygen atmosphere experience increased telomere loss and increased single-stranded telomere breaks, then prematurely senesce. So any drug protecting telomeres against oxidative stress should help, including the "super antioxidant" carnosine [69]. Reducing oxygen in a cell culturing system sometimes yielded cells capable of 3x as many cell divisions [Zs. Nagy, The Membrane Hypothesis of Aging, p.8.].
Aging without Telomere Shortening
Also, note that the fibroblasts of the mouselike dunnart double 170 times, but the animal only lives 3 years, no doubt because the other mechanisms of cellular senescence kill it before replicative senescence does [Zs. Nagy, p.8]. Similarly, it is surprising that the mouse has telomeres > 25,000 base pairs long; typically dying not of replicative senescence, but of mitochondrial failure, which is typical of animals very subject to predation. The relatively safe mouse-like bat lives 40 years, enjoying telomere-controlled longevity. Mice with the hTR RNA component of telomerase knocked out show no old-age effects due to telomere shortening until the 6th generation. Coincidentally, the mouse is more prone to cancer than humans; mouse telomeres are so long that they rarely catch runaway cell division. Telomeres sometimes stop the runaway cell division associated with cancer unless the cancer causes the gene to express telomerase. Syrian hamster embryo cellsexpress telomerase to keep their cellular telomeres long for 20-30 population doublings until they senesce by another mechanism.
"Normal somatic cells are generally telomerase negative, except for bone marrow stem cells." Note that germ cells express the most telomerase, maintaining their telomere length constant, while stem cells senesce more slowly than normal somatic cells because they intermittantly express telomerase [M.Fossel, Cells, Aging, and Human Disease, p.54].
Neygeront and Kinetin
Another Hayflick limit extender is the RNA and B-vitamin complex Neygeront [79], which may boost the number of possible cell divisions by 20%. Kinetin (N6-furfuryladenine), in carefully controlled doses, may be another Hayflick Limit extender. Kinetin, a cytokinin pioneered in anti-aging research by Dr. Suresh Rattan, (also a student of zeatin) is presently being used on skin cells. Mind you, we are sometimes up against the problem: "The senescent phenotype was not prevented... although telomerase activity was induced...", the solution to which we must seek in other sections of this and similar essays. For instance, activating telomerase to lengthen telomeres saves fibroblasts, but not pancreatic islet cells, from senescence.
P16INK4A and the Reversibility of Senescence
P16INK4A can be too high for replicative senescence to be reversed by telomerase activators [Index], but can sometimes be kept within bounds by nerve growth factor, which produces Id-1 helix-loop-helix transcription factor limiting P16INK4A. Note that vitamin A or retinol from carrots, sources of the telomerase inhibitor retinoic acid, reduce P16INK4A expression. However, retinoic acid represses hTERT transcription, so that vitamin A, carrots, retinol, and retinoic acid are to be taken during telomerase activation OFF phases of treatment. P16INK4A is a ubiquitinated protein that is devoured (the rate of ubiquitination depending on P16INK4A density), so that reducing its expression really reduces P16INK4A levels in the cell. See (Ronen Ben-Saadon, Ifat Fajerman, Tamar Ziv, et al, 2004). Resveratrol's heightened SIRT1 expression downregulates P16INK4A expression, retarding the onset of irreversible replicative senescence. Also note that exercise can bring P16INK4A expression down by 2 binary orders of magnitude. High P16INK4A levels are often associated with aging, smoking, and physical inactivity.
Heat Shock Proteins Increase Telomerase Levels
Another method of increasing telomerase levels in the cell is via heat shock proteins, which mediate the assembly of telomerase (GB Morin, DO Toft, JW Shay, WE Wright, MA White, et al., (1999)). Note that HSP90 levels may be elevated for transport of nuclear transcription factors into the nucleus via the action of alpha lipoic acid or via interleukin 6 produced by exercise. HSP90 also guides protein folding as a chaparone.
Telomerase Activation by Nitric Oxide in Endothelial Progenitor Cells
A technique described by Vasa, et. al., used Nitric Oxide to activate telomerase and delay senescence in endothelial cells. See Nitric Oxide Activates Telomerase and Delays Endothelial Cell Senescence and Hayashi, et.al, 2006. Since NO reacts with the superoxide anion to form peroxynitrite, which produces hydroxyl radicals, it is probably a good idea to use the peroxynitrite inhibitor gamma-tocopherol when using NO to promote telomerase activation. Bodybuilding exercisespromote Nitric Oxide synthase production, and in the presence of arginine this leads to Nitric Oxide generation [Books/Nitric Oxide] suitable for telomerase activation and promotion of mitochondrial biogenesis [Books]. Nitric Oxidegeneration is also promoted by broccoli sprouts, genistein, and resveratrol [Links, Books]. Finally, the release of NO in endothelial tissues is promoted by acetylcholine, which may be boosted with ashwagandha, huperzine A, or acetyl L-carnitine. Nitric Oxide release for purposes of telomerase activation might be best promoted in the vascular endothelium by bodybuilding workouts in presence of arginine and ashwagandha, huperzine A, or acetyl L-carnitine as supplements, to which we might add broccoli sprouts, genistein, or resveratrol with gamma tocopherol to neutralize peroxynitrite.
Small Molecule Telomerase Activators
[Index, List, Wikipedia/Telomerase, Links/Histone Deacetylase Inhibitors and Small Molecule Telomerase Activators, Papers, Patents, Books; Index/HDAC inhibitors, Books/telomere homeostasis, Books/telomerase activators, Books/Small Molecule Telomerase Activators, Patents; Endogenous Telomerase Activators; Exercise-Induced Telomerase Activators; History of Telomerase Activators].
Geron Telomerase Activators
Geron [SEC filings, Investor Guide] and a subsidiary TA Therapeutics have announced a small molecule telomerase-activator TAT0002 (TAT2, cycloastragenol), orally bioavailable, presently used in AIDS therapy that may become useful in life extension work, say on T-lymphocytes and on on the skin [article], now to involve telomerase activation therapies[Index] or telomerase transcription-activation methods. Geron has also determined that its telomerase activators [List, Index] promote wound healing. [Geron/telomerase activators: Table]. Concentrations of astragalosides at 1 μg/ml are typical for topical applications or in testing for telomerase activation effectiveness using a telomerase TRAP assay [Books]. Telomerase activity has a half-life of about 24 hours. One of Geron's formulas [See also A' alternate-source version Compositions and Methods for Increasing Telomerase Activity, A'', Hong Kong Univ. formulas and Patent Lens] for a small-molecule telomerase activator is Astragaloside IV (molecular diagram, vendors RevGenetics, Terraternal), which the firm claims is associated with an optimal dosage of 50 mg to 100 mg per day (p.39-40). This looks like an overdose by a factor of 10 or 20, but that may be due to reduced bioavailability of astragaloside IV by itself.
Astragaloside IV is more bioavailable when taken in astragalus root extract, which is typically used to boost the immune system. The bioavailability of astragaloside IV[Article, Papers, vendors RevGenetics, Terraternal], can also be increased by chitosan [Index, Wiki/chitosan, Links/chitosan] or sodium deoxycholate [Index, Links, Books]. The small-molecule telomerase activator TA-65, a product of TA Sciences, primarily composed of cycloastragenol [Index, 81s/6b, Links, Images, Papers, Books] has also been announced by Geronalong with several other formulas based on astragalus root extract molecules [Geron European Patent and Hong Kong University European Patent]. TA-65 was initially prescribed at 5 mg/day, then finally at 25 mg/day. The 5 mg dose was recommended by Geron for cycloastragenol in their European patent (p.39-40). A 1/08/2011 email source quotes a chemical analysis showing that TA-65 is: 5.47 mg cycloastragenol + 0.27 mg astragaloside IV + < 0.01 mg astragenol per serving. (See page 65 for the molecule, and note that "cycloastragenol is the common genuine aglycone of the astragalosides", the smallest of the astragaloside-related telomerase activator molecules, resembling astragaloside IV with its two lower ball-like carbon rings off, as does likewise small astragenol (List). "One-nut" versions of astragaloside IV are described on page 66, cycloastragenol 6-β-D-glycopyranoside and cycloastragenol 3-β-D-xylopyranoside.) TA Sciences at first claimed that TA-65was "one single, potent molecule" from astragalus extract, thus probably cycloastragenol, and not astragaloside IV, which required 50 to 100 mg per day doses to be effective without excipients to improve bioavailability. TA-65 might have been astragenol, the dosage of which was unspecified in the Geron European patent, until chemical analysis emanating from RevGenetics determined that it was primarily cycloastragenol. Geron singles out cycloastragenol as having very low toxicity and excellent bioavailability on page 40 of Compositions and Methods for Increasing Telomerase Activity (or see A' alternate-source version Compositions and Methods for Increasing Telomerase Activity, or A''), and emphasizes a procedure for converting astragaloside IV chemically to cycloastragenol. TA-65 is applied by TA Sciences in staggered fashion 3 months on, three months off, and again three months on during a period of one year in the Patton Protocol.
It seems that after 3 months using TA-41 astragalus extract to test the Patton Protocol, TA Sciences saw 230 base pairs of telomere growth in their early studies [81s/6d]. By May 2012, it seems astragalus extract was more effective in producing increases in average telomere length than TA-65, cycloastragenol, or astragaloside IV, according to studies carried out by the VIDA Institute, Terraternal, and Calvin B. Harley, et al. (2011), in A Natural Product Telomerase Activator As Part of a Health Maintenance Program, Rejuvenation Research, 14(1), 2011. "Results of an informal, but scientifically conducted, several month trial with laboratory analysis of biomarkers, in fact, showed that a combination of whole astragalus root, and natural astragalus extract was an order of magnitude more effective than either cycloastragenol or astragaloside IV at increasing the telomere length of immune system (NK) cells in the blood.... Using Standardised Astragalus Root Extract (+ physical training, meditation, less work, gingko biloba, orlistat and LCHF ).... The median telomere length of my NK cells increased in length by a whopping 1,800 base pairs (in 3 months)." - VIDA Institute. The VIDA Institute recommends taking ginkgo biloba (which makes blood platelets less sticky) and gotu kola (Centella Asiatica) with astragalus root extractto improve its access to the fine structure of the circulatory system, so that it can reach more cells.
About 50-200 telomere base pairs are lost per cell division, a number than can be reduced to from 20 bp to 5 bp by taking antioxidants. Note that humans typically have about 50 cell divisions available at the embryonic stage (except for stem cells), so that such a program of treatment might go on for years. Geron's preferred embodiments in 2005 for a small molecule telomerase activator are based on astragaloside IV, cycloastragenol, astragenol, and astragaloside IV 16-one, although they have named several other effective compounds also obtained from astragalus root extract, including cycloastragenol 6-β-D-glucopyranoside and cycloastragenol 3-β-D-xylopyranoside. Relatively highly bioavailable cycloastragenol may be obtained from astragaloside IV [RevGenetics] or by other methods from astragalus membranaceus. By the Spring of 2011, astragalosides seemed rather less effective than described above, working best as telomerase activators on NK cells, explaining why astragalus is good at deflecting viral infections. Approaches based on several telomerase activators in parallel, plus exercise with HGH secretagogues and support for endogenous telomerase activators, now seem more promising than total reliance on astragalosides or TA-65, based on studies of TA-65 performance by Calvin B. Harley and other leading investigators on data gathered since the spring of 2007. Geron has also described a "formula III" telomerase activator embodiment, ginsenoside RH1 [Index, List, Links, Papers, Patents, Links/ratios, Papers/ratios]. Red Korean Ginseng Extract may include some ginsenoside RH1 [molecule], but on the whole the extract turns out to be a telomerase inhibitor.
Astragalus Preparations from Vendors
In 2007 I thought the best and safest commonly available medicine for telomerase activation was GAIA Herbs Astragalus Root Extract featuring 30 drops/mg of astragalosides, given at 5 mg of astragalosides/day during 2-week activation time cells alternating with 2 week periods of telomerase inhibition with telomerase inhibitors like garlic, curcumin, resveratrol, quercetin, silymarin, melatonin, vitamin E, green tea EGCG, and fish oil's EPA. Since GAIA Herbs Astragalus Root Extractwas no longer available at 1 mg astragalosides per 30 drops, I at first (2008) substituted 1200 mg/day of Solaray Astragalus Root Extract to cover the 5 mg of astragalosides, which requires 6 x 200 mg capsules per day in a cyclic protocol featuring 15 days on, then 15 days off. By December 2009, I had to substitute 5 droppers of Herb Pharm Astragalus Root Extractplus 6 x 200 mg capsules of Solaray Astragalus Root Extract to approach the 5 mg of astragalosides per day requirement, adding 4 to 8 capsules of Natural Balance Chitosan to improve bioavailability. This was for the first two-week part of the cycle, which features telomerase activation. During the 2nd part of the month-long cycle, telomerase inhibitors are used instead, as described above. Although this exceeds the recommended dose according to Solaray, I believe from toxicologystudies [Index/Astragalus Extract] that it is safe enough. By 2011 I was able to obtain Astragaloside IV at 100 mg/day from vendors like RevGenetics and Super Smart, and cycloastragenol at up to 25 mg/day from several vendors.
By 2012 I dropped astragaloside IV and cycloastragenol, returning to 1/2 bottle of GAIA Herbs green label astragalus root extract per day (15 x 30 drops = about 15 mg astragalosides at the time) with 50 x 500 mg = 25 g) of NOW astragalus root powder in capsules, as it became clear that this was both more effective and more inexpensive. I put 3-5 droppers of the 1/2 bottle of GAIA Herbs green label astragalus extract into my scalp to stimulate hair follicle cycles. Then the cost is approximately 15 x ($10.50/2) = $78.75/month for the astragalus extract and ((50) x $5.91/100) x 15 = $44.32 for the astragalus root powder capsules, for a total of $123.08/month. Using just 5 mg of astragalosides/day from the extract, I might have cut the cost to ($78.75/3) + $21.00 = $47.45/month. Using exclusively NOW astragalus root powder capsules at 33 grams/day, the maximum dose of astragalus root powder used in Chinese medicine, I would have spent 15 x (66 caps) x ($5.91/100 500 mg caps) = 15 x $3.90/day = $58.51/month. To this one would typically add 3-5 droppers (30 drops/dropper = 1 mg astragalosides) per day of the GAIA Herbs green label astragalus extract for the scalp. This would cost 3-5 droppers x ($10.50/30 droppers per bottle) x 15 days, or $15.75/month for 3 droppers/day in 15 days of a month, up to $26.25/month for 5 droppers/day for 15 days of the monthly cycle. Then the cost would be less than $84.76/month for 33 grams/day of astragalus root plus a deluxe astragalus root extract scalp rub. See the complete current program for 2013, which features a number of telomerase activators and booster supplements for them.
Combinations of Telomerase Activators [List]
Combinations of other telomerase activators also became available, such as RevGenetics Astral Fruit NF and custom combinations including Fenugreek extract for diosgenin and 4-hydroxyisoleucine for an insulin boost to provide improved access by telomerase to telomere t-loops by phosphorylating tankyrase 1 for stripping TRF1 telomere loop closure proteinfrom telomeres in the presence of a NAD+ substrate provided by early morning niacinamide (nicotinamide). See Age Transformation for a logbook of this experiment. Telomerase inhibitors and telomerase activators are not to be taken at the same time. Reconstructing cellular telomeres using activation of telomerase closes chromosomal telomere t-loops in senescent cells, restoring the youthful phenotype and patterns of gene expression at a rejuvenation rate of up to 0.75 years per month (9 years/year, or 460 bp/year of telomere growth using the cyclic Patton Protocol). Another alternative is Herbal Remedies Astragalus Root Extract 1.25 mg astragalosides per 250 mg cap, via Nature's Way, ( Standardized 0.5% Astragalosides ), 60 VCapsules per bottle, incuding Astragalus, dried extract 250mg (root) 0.5% astragalosides, with Astragalus (root) 250mg. Four capsules yield 5 mg astragalosides plus 1 gram of astragalus membranaceus root, which improves bioavailability of astragalosides. Note that Ginsenoside RH2, a telomerase inhibitor, may also be present in ginseng extracts, in addition to telomerase activator ginsenoside RH1. This may be why Korean Red Ginseng is a telomerase inhibitor (31). Note that the ginsenosides [Index, Links, Books] are distinct from the astragalosides, being triterpene saponins [Index, Books] from ginseng [Index, Links, Books, in circulation].
One starting material from ginseng is panaxatriol. Geron also names astragalosides A, 1, 2, 7, and astraverrucins I and II, which can be obtained from Astragalus Verrucosis, as telomerase activators. TA-65 "is a naturally occurring molecule extracted from the well known Traditional Chinese herb Astragalus membranaceus [Index, LifeExtension, Solaray, GAIA Astragalus extract, 1 mg of astrogalosides per 30 drops]", also called Milk Vetch Root, or Huang-qi (Yellow Leader) [81s/6b]. However, there are indications that astragalus extract works best to increase telomere length in Natural Killer cells. Geron seems to have tested drugs and nutraceuticals known to boost the immune system in its search for small molecule telomerase activators [List], as both astragalagus root [Index] and ginseng root [Index] had been used in traditional medicine for the immune system. Both contain saponins [Index]. Perkins Coie has recently filed a patent application on behalf of Geron's Chief Scientific Officer Calvin B. Harley [Index, Forbes/Calvin B. Harley, Papers/Calvin B. Harley, Books, Portrait] for anti-aging skin creams using astragalosides. Liposome delivery systems [68] in connection with small molecule telomerase activator delivery look quite interesting.
Cut-Rate Telomere Therapy with Fenugreek, Black Cohosh, Exercise, HGH Secretagogues, Garlic, and Onions
Perhaps Fenugreek seed alone at 3 grams/day just before bedtime for 20 days of the month can preserve us at a specific age. Then telomere length might be held constant for just $5.00/month for 100 caps of 610 mg/cap Fenugreek seed from Wal-Mart, taken 5 caps/day for 3 grams/day for 20 days. Black cohosh for 25 days at 4 caps/day from a 100 caps for $5.00 bottle might also help stabilize telomere length at a reasonable price, or the two could be combined. Thus low-cost telomerase activators exist that may allow us to hang on as well-preserved cut-rate fellow travelers for 5 to 10 dollars/month, even when we can't afford $100/month for basic rejuvenation treatment with astragalus formulations. Exercise can be free and is a source of endogenous telomerase activators that can be boosted with nutraceuticals such as whey protein. HGH secretagogues in combination with exercise may be most useful, as virtually human cells have about the same density of HGH receptors in their cell membranes. Garlic [List] improves the Hayflick limit for dermal fibroblasts, and onions contain quercetin [List], which has some telomerase inducing power.
Telomolecular Nanotechnologies
Another firm, (now-defunct) Telomolecular Nanotechnologies [SEC data 11/7/06], also developed therapeutic products based on telomerase activation, and was developing products including rejuvenating skin creams based on telomerase activation. According to now-defunct Telomolecular Nanotechnologies (Corporate Video), youthful "telomerized cells" seem immune to free radical damage and glycation, due partly to the telomerase position effect described in subsequent section. "...Cells with sufficiently elongated telomeres energetically produce, in high levels, proteins like catalase, superoxide dismutase, glutathione, Ku, collagen, elastin and many other proteins important in tissue formation, cell repair, and antioxidation, that become scarce as telomeres shorten." (See Overexpression of Telomerase... by Armstrong, G. Saretzki, and Peters, et. al.)
DNA Rolling Nanocircle Encoding
Telomolecular Nanotechnologies proposed DNA nanocircles for telomere re-extension [Telomolecular Nanotechnolgies/nanocircles, Amazon, Links, Images, Papers, Patents, Books]. Produced by a DNA synthesizer, DNA rolling nanocircle encoding for telomere rejuvenation was invented by Dr. Eric Kool (article) of Stanford University. See Dr. Kool's patent, Telomere-Encoding Synthetic DNA Nanocircles, and Their Use for the Elongation of Telomere Repeats(More). However, DNA nanocircles for telomere extension are not ready for internal use at this time, but are only used for special applications and in the study of ALT mechanisms in cancer. "Telomolecular believes that nanocircles might work efficiently in living animals."
Direct hTERT Delivery to Cells
Another firm, Phoenix Biomolecular, has specified a cell penetrating peptide scheme to deliver hTERT directly to cells. It is subsequently necessary to phosphorylate hTERT then for transport into the cellular nucleus. Liposome preparations that enter the cell via endocytosis are promising both for large molecules and plasmids. Plasmids in which Green Fluorescent Protein is driven by a copy of the hTERT promoter on the plasmid may be used to guide cancer surgery, and finite-lifetime plasmids expressing C-myc may be used to enhance hTERT transcription levels.
hTERT mRNA Delivery to Cells
Fast telomere extension using HPLC-purified, nucleoside-modified hTERT mRNA to escape from the innate immune system, uses mRNA with amplified poly(A) tails that gradually shrink to a minimum length before the mRNA is consumed by an enzyme. The therapeutic hTERT mRNA may be transfected in liposomes. It is capable of lengthening telomeres in 6 days by the amount that telomeres shrink in 15 years of aging, so that it should be useful for stem cell transplants and rejuvenation.
Sierra Sciences Identifies 858 Telomerase Inducers
Sierra Sciences [Wikipedia] is also working to develop telomerase activation techiques [Patents]. More than one small-molecule telomerase activator [Links, List] has been found. I list 178 substances under investigation as telomerase activators. Some of them are hTERT transcription activators binding to the hTERT promoter producing hTERT mRNA transcripts and others are hTERT phosphorylation kinases (AKT) that phosphorylate hTERT in the cytoplasm to import it to the cell nucleus. Both seem to produce telomerase activity. In addition, there are HDAC inhibitors like Tricostatin A and CGK 1026 that improve hTERT transcription by expanding chromatin, and cofactors such as HSP90 that are required for hTERT assembly. Sierra Sciences claims to have identified 858 telomerase activity-producing substances after screening 254,593 compounds, more than the 178 telomerase activators I have listed, some of which were identified from Product B. The relative speed with which alternative telomerase activators can be used to achieve rejuvenation is being measured by Sierra Sciences, TA Sciences, Terraternal, RevGenetics, and other firms. For Sierra Sciences (President William H. Andrews PhD) and IsAGenix telomerase inducer test results for 27 phytochemical extracts and associated Product B telomere enlongation results, see the Product B PatentScope.pdf developed by Master Formulator John Anderson. Also see telomerase activator (121) Product B and The Product B Explorer.
Endogenous and Exercise-Induced Telomerase Activators
Note that I have also identified endogenous telomerase activators and 15 exercise-induced telomerase activators together with supplements for promoting their expression. Note that cyclic AMP from exercise and certain supplements such as forskolin can lower caveolin-1 expression in senescent cells by sequestering FOXO factors away from the cellular nucleusin a way that can produce recovery from cellular senescence plus telomere enlongation.
HDAC Inhibitor Telomerase Activators
See papers on telomerase activation, for instance by histone deacetylase inhibitors [Index, Links, Images, Video, Papers, Patents, Books]. "Treatment with tricostatin A (TSA) [Index, Wikipedia, Books, Links, Epigenetic Protocols, chap.8, Future_Medicine/article, article2, anticancer, elevates Hsp22 level with life extending effect] induced significant activation of hTERT mRNA expression and telomerase activity in normal cells, but not in cancer cells." Histone deacetylase inhibitor trichostatin A "activates the hTERT promoter in normal cells", thus enabling telomerase synthesis. I stated above that resveratrol is an activator for SIRT1, a histone deacetylase enzyme [Index/SIRT1, Index/HDACs, Links, Images, Papers, Patents, Books, LifeExtension], the activation of which results in gene silencing that keeps DNA more tightly wound on histone cores, preventing eccDNA formation. Perhaps resveratrol can be used as a SIRT1 gene silencer to shut down telomerase transcription from hTERT following treatment with a small-molecule telomerase activator like tricostatin A(TA/Tricostatin A), a histone deacetylase inhibitor which is genotoxic at some dosages, however. Resveratrol turns out to activate hTERT protein in the cytoplasm via hTERT phosphorylation with the AKT protein kinase (Index/AKT), enabling the import of cytoplasmic hTERT protein into the nucleus, but not improving the number of hTERT mRNA transcripts via activation with a nuclear transcription factor such as HIF-1 or C-myc dimerized with Mad, a helix-loop-helix transcription factor. Both bind to the same type of 5'-CACGTG-3' E-box promoter site on the hTERT promoter. CGK 1026 [Telomerase Activators/CGK 1026, Index, Links, Papers, ChemBank/CGK 1026 molecule], discovered in 2004, is said to "derepress" hTERT expression, and has been used as an orally bioavailable substitute for Tricostatin A. CGK 1026 is thought to inhibit the recruitment of HDAC [Links/HDACs] into E2F-pocket protein complexes assembled on the hTERT promoter. It is described by several sources: Linscott's Directory of Immunological and Biological Reagents, as catalog # 565730, Merck CGK 1026 - Order # 565730-5MG, and EMC Biosciences CGK1026. See also SP1 on the hTERT promoter (hTERT promoter/SP1) for repression and derepression of hTERT transcription. A more detailed look at the situation shows that some commonly available foods contain histone deacetylase inhibitors that may prove valuable in life extension via telomere extension by telomerase activation.
For instance, diallyl sulphide (garlic) and sulphoraphane (broccoli) are zinc-activated (meats, seafood, oysters) class I histone deacetylase inhibitors (HDAC inhibitors). Zinc enhances hTERT transcription by upregulating Bcl-2 (a telomerase activator) and may be used up to 45 mg/day. Another allyl compound from garlic or deodorized garlic [Wikipedia/garlic, Links, LifeExtension] that is more active as a histone deacetylase inhibitor than allyl sulphide [Links] is allyl disulphide [Links], and a still stronger one is allyl mercaptan [Links]. I note that allicinfrom garlic inhibits telomerase expression in cancer cells, often inducing apoptosis. Garlic extends the Hayflick limit in dermal fibroblasts, so that allicin metabolites diallyl disulfide and allyl mercaptan are thought to improve hTERT mRNA expression in normal cells. Note that overdoses of garlic and allyl compounds can have toxic effect; consuming 5 garlic cloves in a tomato soup with chopped onions can induce diarrhea. Butyrate [Index, Links] is also a histone deaceylase inhibitor (10) or chromatin-remodeling factor that may lengthen telomeres by enabling telomerase transcription and is known to extend the life span of Drosophila 40%. Sodium butyrate [Index, Images] is an HDAC inhibitor available now in pill form that may be able to accelerate hTERT transcription by keeping chromatin expanded and available for transcription, so that it might be taken together with astragalosides or other telomerase activators. Sodium butyrate improves the transcription of Sp1, which markedly improves the expression of the telomerase activator epiregulin, a ligand of the epidermal growth factor receptor.
"FR901228" (Romidepsin) is a typical new histone deacetylase inhibitor [Links, LifeExtension] being investigated by Japanese scientists that is known to activate telomerase transcription. Resveratrol (red grapes, Japanese Knotweed root) is a class III histone deacetylase activator via its ability to turn on SIRT1 deacetylase enzyme, collapsing chromatin and inhibiting transcription of hTERT mRNA. However, resveratrol phosphorylates hTERT molecules in the cytoplasm for import into the nucleus, activating telomerase. Resveratrol is sometimes described as a deacetylation activator, and activates the gene SIRT1, yielding the corresponding NAD-dependent SIRT1 deacetylase enzyme for gene silencing via chromatin condensation. NAD, incidentally, is found in tuna, and may be synthesized in the body from niacinamide (nicotinamide), a form of vitamin B3. By 2011 we were using TA Sciences TA-65, astragaloside IV, cycloastragenol, astragalus extract, or astragalus root, in parallel with other telomerase activators [Index, List] such as Nitric Oxide and fenugreek extract to to cover our telomere elongation requirements, with feedback from telomere length testing[LifeXLabs/Telomeasures, Links, Papers, Patents, Books, LEF]. The histone deacetylase inhibitors prepare chromatin DNAfor transcription with RNA polymerase, dissociating the histones from DNA prior to transcription. Some, like sodium butyrate, may be useful for accelerating transcription. There are 4 types of covalent modifications of histone proteins - acetylation, phosphorylation, ADP-ribosylation, and methylation. Histone deacetylation inhibitors allow chromatin to expand and become transcriptionally active, sometimes activating telomerase. Histone deacetylation causes compaction of chromatin, silencing genes. Histone acetylation expands chromatin, while histone deacetylation compacts it.
HDACs may activate p53, while activation of SIRT1 (human SIR2) with resveratrol prevents p53 activation with chromatin compaction and gene silencing, causing extended life span, since telomere shorting itself finally induces a p53-dependent DNA damage detection pathway leading to the senescent state of the cell. Furthermore, resveratrol activates telomerase by phosphorylating cytoplasmic hTERT for import into the nucleus, also tending to inhibit senescence. What we need most are hTERT transcriptional activators that make the correct sections of chromatin transcriptionally active for hTERT mRNA production, such as astragalus root (33 grams/day), astragalus root extract (for application to the scalp), colostrum solution (for transdermal application), hawthorn extract, bacopa (1-2 grams/day), fenugreek seed (3 grams orally before bedtime), arginine (5-10 grams/day for Nitric Oxide), whey protein (for HGH), DHEA (4 x 50 mg/day for higher testosterone), and exercise. HDAC inhibitors assist hTERT transcriptional activators by expanding chromatin and may themselves induce transcription of hTERT mRNA.
Interleukin 2
A larger molecule of about 15,500 Daltons (still capable of passing through the nuclear pore) worth exploring is the cytokineIL-2 or interleukin 2 [Index, TA/Interleukin 2], a "lymphokine" which augments the expression of mRNA for human telomerase in T-cell lymphocytes. IL-2 "has been approved by the Food and Drug Administration (FDA) for the treatment of cancers (malignant melanoma, renal cell cancer), and is in clinical trials for the treatment of chronic viral infections, and as a booster (adjuvant) for vaccines." - [Wikipedia]. Interleukin 2's telomerase-enhancing role in T-lymphocytes has been noted by other investigators. [See IL-2 source BD, Links/IL-2]. There is evidence that DHEA acts as a physiological regulator of interleukin-2 biosynthesis (Yu, 1995, citing in Arking, The Biology of Aging, p.233.), so supplementation with DHEA may help interleukin-2 maintain T-lymphocyte telomeres.
Estrogens
Preliminary data suggest estrogen [Telomerase Activators/Estrogen] activates telomerase in T-lymphocytes [Handbook of Models for Human Aging, p.37]. Furthermore, "estrogen and its receptor activate telomerase in estrogen-responsive cells through the estrogen response element sites in the hTERT promoter".
Epithalon Peptide
Another group in Russia at the St. Petersburg Institute of Bioregulation and Gerontology announced in 2003 that Epithalon Peptide [Index, Telomerase Activators/Epithalon Peptide, epitalon, aka epitalon], a small 4-peptide protein made from the sequence alanine, glutamine, aspartic acid, and glycine, (Ala-Glu-Asp-Gly), activates telomerase and extends telomeres [Links, Papers, Original Paper, Biogerontology article 1, article 2, epithalamin articles, sources, sources2, sources and costs]. Epithalon peptide has been injected 5 times weekly into mice, resulting in 34.2% prolongation of life span in mice without cancers. Liposomal or sublingual (under the tongue) administration is probably possible with such short peptide molecules, and perhaps it can be topically applied like a skin cream, taken sublingually under the tongue, in buccal fashion in the pouch of the cheek, or otherwise.
HGH and IGF-1 as Telomerase Activators
Mice overexpressing IGF-1 showed higher levels of phospho-Akt [Links, Books] leading to increased telomerase activityand more effective cardiac stem cells, creating a better supply of new myocytes for the heart. (Torella et.al., 2004, cited by Arking). Telomerase activity can be stimulated by improving our levels of IGF-1 (Telomerase Activators/IGF-1) by direct input of the hormone or by stimulating it via exercise in the presence of amino acid stacks [Human IGF-1 stimulator, Links/IGF-1, Books].
I note that Human Growth Hormone (HGH) has been shown to upregulate telomerase in ovaries and in liver cells (hepatocytes). [Links, Books]. Furthermore, HGH (which improves transcripiton of hTERT mRNA) is partially converted by the liver into IGF-1 (which phosphorylates hTERT protein for import into the nucleus). Note that HGH and IGF-1 are both upregulated by exercise, as are at least 12 other endogenous telomerase activators and at least 15 telomerase-activating human growth factors found in growth factor skin creams. On the other hand, an antagonist of growth hormone-releasing hormone has dramatically decreased telomerase activity [Links, Papers, Books, Books2] in experimental xenografted transplants [article]. Telomerase activation has been achieved in cord blood using IGF-1 in the presence of PHA (phytohaemagglutinin; 1 μg/ml, Sigma, St Louis, Mo., U.S.A.). Note that while attempting telomere extension via a growth hormone strategy for telomerase activation [Patents], one should avoid ingesting telomerase inhibitors, which can sometimes be produced in the body from high-polyphenol diets. However, some telomerase activators for normal cells (such as silymarin) behave like telomerase inhibitors when applied to cancer cells.
Ecdysone
Evidently, there has also been some progress in hTERT activation using the insect hormone ecdysone (TA/Ecdysone, muristerone) to induce transcription from hTERT and cellular immortalization in human fibroblasts [article]. Finally, I note that US Patent 6787133 has been granted to Geron for a procedure to purify telomerase for the purpose of identifying telomerase activators and telomerase inhibitors.
Increased telomere length, more telomerase activity, and active meristematic stem cell presence have been identified as important factors in the long life spans of 2000-5000 year old bristlecone pine trees [106]. Furthermore, inducing more telomerase activity in embryonic stem cells seems to improve their defenses. Although telomerase expression is up-regulated in cancer, and used to detect cancer, the gene for telomerase is not an oncogene and its activation does not induce cancerous growth deregulation by itself. Inhibition of telomerase in human tissues helps suppress run-away cancer cells by inducing massive apoptosis in tumors, so telomerase activation treatments to lengthen our telomeres for life extension purposes [Age Transformation, Cyclic Telomerase Activation] should be periodic and short-term, or done ex vivo prior to an adult stem cell transplant, and not continuously in vivo. Telomerase activators are weakly tumor promoters, as they inhibit telomerase inhibition as an anticancer defense. TA Sciences proposes using their TA-65 telomerase activator 3 months on, 3 months off, and 3 months on again for a year (the Patton Protocol).
Cyclic Telomerase Activation with Readily Available Nutraceuticals
I expect that monthly cycles of 2 weeks of telomerase activation followed by two weeks of telomerase inhibition plus treatment with anticancer nutraceuticals is an adequately safe approach. This may allow stronger telomerase activation than is feasible with the Patton Protocol. In the future we may prefer to use Product B, astragalus root extract with chitosan, purslane extract, black cohosh, colostrum (for telomerase-activating growth factors and IGF-1), ginkgo biloba for HIF-1, fenugreek extract (for HIF-1, testosterone, diosgenin, and insulin), arginine for Nitric Oxide, alpha-GPC with exercise for HGH, Nerve Growth Factor boosters (acetyl L-carnitine, huperzine A, carnosic acid from rosemary) to promote ID-1 helix-loop-helix transcription factor, or other telomerase activators under investigation to lengthen our telomeres, along with telomerase-activating foods like whey protein (for HGH), creatine monohydrate (for IGF-1 and to boost cAMP), apples and onions (for quercetin), and garlic (for HDAC inhibitors improving hTERT mRNA expression). Some of these, such as astragalus root extract, colostrum, and black cohosh, may be taken orally and also rubbed in to the skin and scalp directly for best effect, rubbed into the gums and oral cavity, or even applied in suppository form (astragalus extract in glycerin). Telomerase-activating drops for the eyes (similar to carnosine drops for the eyes) should be researched. Perhaps colostrum spray mist would be suitable, although it must be refrigerated and verified as useful and safe. Essential oil of rosemary may have telomerase-activating effect in the sinuses via nerve growth factor activation of ID-1, where we might also consider using colostrum spray mist or astragalus extract nasal spays.
Hilariously, no mouse has ever died from telemere shortening, or suffered from a shortage of telomerase, however. In mice the dominant aging mechanism seems to be mitochondrial aging via lipid peroxidation.
On the other hand, relatively cancer-resistant humans can die from telomere shortening; as telomere length goes to zero once-mitotic bone marrow, human skin, liver, colon mucosa and lymphocyte cells become senescent and chromosomally unstable. Lymphocytes then suffer irreversible cell cycle arrest. Cell death follows, sometimes after a lengthy delay. Progress on small molecules for telomerase activation is also proceeding from research on plants. Recently it has been shown that telomere shortening is aggravated in each cell division of mitotic cells by high homocysteine levels, so that a homocysteine shield [Links, Images] featuring TMG (trimethyglycine) 500 Mg - 720 mg, folic acid 400 Mcg, vitamin B6perhaps 25 Mg, and vitamin B12 250 Mcg is effective at preserving telomere length, in addition to preserving the endothelial cell linings of the veins and arteries of the heart and the brain and fending off atherosclerosis. [See Dr. Phillip Lee Miller, Life Extension Revolution.] Both antioxidant treatment preventing free-radical-induced single-strand DNA breaks (repaired by Cat's Claw extract (AC-11) [Index] along with double-strand breaks and photochemical DNA damage) and homocysteine treatment featuring a homocysteine protection formula preventing more telomere loss per cell division tend to preserve telomere length, so that some centenarians seem to have rather long telomeres.
Another possibility for lengthening telomeres, Alternative Lengthing of Telomeres [Links/ALT], may be implemented with DNA nanocircles, which seem to be generated in cancer cells from long telomeres via recombination of long telomeres achieved by homologous DNA repair. Researchers picture the small telomeric circles forming through the resolution of an intratelomeric strand invasion resembling a t-loop during homology-directed repair (HDR) of telomeric DNA usually repressed by the POT1 component of the telomere nucleoprotein complex shelterin, also termed the telosome. Two recent papers demonstrated that human alternative lengthening of telomere (ALT) cells have abundant t-circles similar to Telomolecular Corporation's (Corporate Video) DNA nanocircles, pointing to their potential role in promoting telomere replication in the absence of telomerase. "Analysis of telomere restriction fragments from human cells that rely on ALT for telomere maintenance revealed that they possess telomeric tracts that are extremely heterogeneous in length, ranging from undetectable to abnormally long (Bryan et al., 1995), which would also point towards a recombinational origin (see also Henson et al., 2002)." Intense interest in the ALT pathway exists partly because it is a stumbling block to the application of telomerase inhibition as cancer therapy. Without telomere extension via small molecule telomerase activators or synthetic nanocircle ALT, however, it seems death is finally certain, as telomeres will be consumed eventually otherwise, destroying T-lymphocytes in the immune system, microglial cells in the brain associated with clean-up operations, the skin, the lining of the internal organs, and all other mitotic cells including the lining of the colon.
I might add that the consequence of cloning an animal from a mature specimen with shorter-than-embryonic telomeres is that the clone dies young, as in the case of Dolly the sheep, the first cloned mammal to make headlines. To successfully clone an adult requires pre-extension of telomeres prior to nuclear transfer or cloning from embryonic tissues, otherwise, the cloned offspring may die ahead of time [Books]. In practice, cattle are typically cloned from embryonic or fetal cells to avoid the short-telomere early death syndrome. However, recently we have found out that cloning from senescent cells at the end of their lifespan can produce strong telomerase activation in a way that produces potentially very long-lived offspring in cattle with telomeres longer than ever! It is also noteworthy that people having a longer telomere length (upper half of the population) live longer than those in the lower half of the population, who have 1.86 times the mortality rate, with 3.2 times the heart disease and 8.5 times the infectious disease of the long telomere upper half. [Cawthon, et.al., 2003, cited in The Biology of Aging by Robert Arking, p.442.]
Breast Cancer, Cell Division, and Astragalus Root
The average age for breast cancer in males is 67, and in women 62, afflicting 1 in 8 women over a lifetime. During each menstrual cycle, estrogen together with other ovarian hormones signals cells in the breast to divide and multiply. Breast enlargement involving estrogens and other ovarian hormones in males or females may result in cell divisions sufficient to drive cells closer to the Hayflick limit, when they become senescent and genomically unstable after telomeres become uncapped, thus cancer-prone. Thus anti-senescence drugs like the small-molecule telomerase activators in astragalus root extracts or silymarin may be useful in preventing breast cancer arising from genomic instabilities in breast cells that have divided many times and eventually become senescent ahead of other cells as a consequence of estrogen and ovarian hormone signaling. I note that telomerase activator black cohosh improves the stability of breast tissue against cancer. Busty aging mates might take astragalus root extract to ward off breast cancer before it is observed, it seems.
We wonder if the term "astragalus" itself did not signify originally this set of patients that could benefit from the use of the herb. "Recent studies show that more than 90% of all cancer is caused by critical telomere shortening [Links, Images, Papers, Books; Links/cancer due to critical telomere shortening, Books], for example, in 97% of premalignant epithelial lesions [Images] critical telomere shortening is observed (Meeker, John Hopkins University 2005, Papers)." - Telomolecular Corporation SEC statement. In the breast, progesterone also acts as a chemical messenger that tells breast cells to divide, hence progesterone and estrogen are applied in female transformations which can become ultimately dangerous when finally cells approach the Hayflick cell division limit. [Links/breast cancer, Books, Wikipedia, Index]. Chromosome 17 has short telomeres [Links] implicated in breast cancer. Perhaps astragalus extract in glycerin or in liposomes would be useful if applied topically to the breast, in addition to astragalosides or other small molecule telomerase activators such as black cohosh taken orally to apply telomerase activation in a cyclic manner for improving stem cell and tissue genomic stabilityagainst malignant transformations.
The Telomere Positioning Effect & Youthful Patterns of Gene Expression
Also see the telomere positioning effect [Links, Images, Papers, Patents, Books, LifeExtension]. Long telomeres silence genes close to the telomere, and as the telomere shrinks genes proximate to the telomere are more expressed. Therefore to obtain youthful patterns of gene expression in mitotic, dividing cells, it is desirable to maintain long telomeres [Papers, Patents Books]. Telomeres are transcribed to produce TERRA telomeric RNA, starting from subtelomeric DNA and moving toward the end of the chromosome, when telomeres become uncapped. This reduces the telomere position effect inhibition of the transcription of genes near the telomere, and may improve telomeric DNA repair, since some DNA repair is only done during transcription. A good deal of work is now going on to identify telomere-shortening-sensitive-genes (TSSG) [Links, Papers, Patents, Books]. I note that the shortest telomere on human chromosome 17p [Books, Wikipedia/Chromosome 17, Ornl, Genes & Disease; GeneCards/P53] at 17p13.1 seems to be fairly close (within 7,512,445 bp out of 80 million bp) to the gene for the tumor suppressor gene p53, so that more tumor suppressor p53 protein should produced when this telomere gets sufficiently short. This way shorter telomeres would work together with the tumor-suppression system and apparatus for stopping the cell cycle and to induce the senescent state of the cell when telomeres shorten. See Links/the telomere position effect and tumor suppressor gene p53. "The level of p53 protein increases in near senescent cultures" of cells [Vaziri and Benchimol, 1996].
It turns out that subtelomeric DNA contains genes for zinc fingers [ZFC, Wikipedia, Links/zinc fingers, Books] and olfactory receptors [Books] that should be more actively transcribed when telomeres are short. Zinc fingers [Books, Books/zinc fingers in cellular senescence] may be transcribed in clusters that activate or deactivate promotors for genes like fingers applied to a piano keyboard. Their telomere position effect activation in subtelomeric DNA when telomeres become short may directly impact gene expression to produce some of the observed aging effects. Perhaps someday RNA interference [Images, Papers, Patents, Books], or RNAi, may be applied to oppose undesirable effects caused by the expression of mRNA controlled by zinc fingers in subtelomeric DNA. Genes involved in the wrinkled skin, graying hair, and healing abilities may be involved, as Geron has shown that these are improved by lengthening telomeres [Wikipedia/Telomerase]. "Cells with sufficiently elongated telomeres energetically produce, in high levels, proteins like catalase, superoxide dismutase, glutathione, Ku, collagen, elastin and many other proteins important in tissue formation, cell repair, and antioxidation, that become scarce as telomeres shorten." [From Telomolecular Corp/case studies, see also LifeExtension/Telomere Control and Cellular Aging, the papers of Dr. Woodring Wright and Dr. Jerry Shay.]
"As the hTERT gene is only a few hundred kilobases from the end of chromosome 5p [Books], one could speculate that TPE (silencing) of hTERT limits the maximal length of human telomeres during embryogenesis." [JW Shay & WE Wright, 2005.]. (In earlier 2002 work [Cong, Shay, and Wright, 2002], the hTERT gene was stated to be the most distal gene on 5p, but 2 million base pairs from the telomere, so that the telomere position effect would have little effect on hTERT.) Evidently, the telomere position effect should cause the body to automatically resist aging by more strongly activating telomerase as we age, a tendency we must supplement to achieve a longer-than-usual lifespan. Perhaps proteins mediating telomerase assembly such as p23 and Hsp90 are less available as time goes on. The location of the hTERT gene (TERT) in other species with different lifespans becomes an interesting question. Species may be engineered in the future with extra distal hTERT genes on other chromosomes to extend their life spans. Perhaps the telomere position effect is also involved in thymic recovery and the withering of the thymus gland, which is involved in T-lymphocyte production and connected to the immunological theory of aging (11). Recovery of thymus gland function is sometimes observed when a patient is treated with 10 grams of arginine per day to produce Nitric Oxide, a telomerase-activating vasodilator.
On the other hand, as we shall see according to the Membrane Hypothesis of Aging (13), to obtain youthful patterns of gene expression may also require modifying the cell membrane permeabilities (modified by cross-linking of membrane-imbedded proteins) to ionic species such as potassium that modify the dehydration, colloidal properties, and density of cellular material, factors which should apply to both mitotic and non-mitotic cells. In dense, dehydrated cells enzyme reactions are inhibited, so that protein turnover and protein synthesis are retarded, tending to produce more ceroid wastes and lipofuscinthat choke the cell and increase free radical production, eventually leading to inflammation and processes involving proinflammatory cytokines that are ultimately poisonous to the cell. Also, deproteinization of chromatin restores its youthful low DNA strand separation temperature, so that restoring youthful patterns of gene expression [Books] may be assisted by undoing protein cross-links or other protein properties in chromatin that inhibit DNA strand separation [Books] and gene expression [Books, Books/chromatin and gene expression]. See Maria A. Blasco on the telomere position effect in Maria A. Blasco (2007), The epigenetic regulation of mammalian telomeres, Nature, April 2007.
Telomere Capping Proteins
[Index/Telomere Loop Control Proteins, Index/Mammalian Telomere Protein Factors, Telomere Extension by Telomerase, Telomerase Components in Cell Signaling, Links, Papers, Books, Links/capping supplements].
The telomere t-loop may be opened to make it accessible to telomerase by phosphorylating tankyrase 1 with insulin using insulin-boosters such as Gymnema Sylvestre, Fenugreek Extract, Fenugreek seeds, or 4-hydroxyisoleucine. This may be amplified somewhat in the presence of dextrose. Tankyrase 1 levels may also be boosted with niacinamide (nicotinamide, a form of vitamin B3). Tankyrase 1 uses NAD as a substrate in poly(ADP-ribosylation) of TRF1, so NAD supplements[Links] may be useful when applying tankyrase 1 to enable telomerase access to the end points of otherwise closed telomere t-loops. Note that nicotinamide elevates NAD+ (NADH, Nicotinamide Adenine Dinucleotide) levels, but should be taken early in the morning on telomerase activation days to build up NADH levels, since it has inhibiting effect on PARP enzymes such as tankyrase 1 that I prefer to phosphorylate via evening doses of insulin-boosters such as Fenugreek extract. Tankyrase 1 opens the telomere t-loop via telomeric PARP activity involving poly(ADP-ribo)sylation with a NAD+ substrate that strips the telomere of the telomere binding protein TRF1. Once the loop is open, the telomerase holoenzyme can access the telomere to extend it.
Uncapped telomeres [Links, Images, Papers, Patents, Books] terminate in a single-stranded overhang 100-300 nucleotides in length bound to the telomere loop along its length by POT1 and at its starting point by TRF1. Dr. Yie Liu's lab at the National Laboratory of Molecular Gerontology has illustrated that the proteins PARP1 [Index, Links, Images, Papers, Patents, Books] and MSH2 [Index, Links, Papers], a DNA mismatch repair protein, play a role in maintaining telomere capping function [Links, Images, Papers, Patents, Books] in which a telomere t-loop configuration many thousands of base pairs long is believed to protect chromosome ends from being recognized as broken DNA. As I note in section 10 on DNA Repair, the maximum life span of 13 species is directly correlated to the activity of poly (ADP-ribose) polymerase (PARP) in mononuclear leukocytes. Disruption of the telomere loop [Images Papers, Patents, Books] and subsequent exposure of the 3' overhang represents the uncapped state of telomeres. The ability to mask telomeres from being recognized as damaged DNA is crucial to maintaining normal cellular function, as uncapped telomeres directly associate with many DNA damage response proteins [Links, Images, Papers, Patents, Books] in telomere-initiated cellular senescence. Telomere integrity depends on the ability to maintain telomere length and/or the ability to mask telomeres from being recognized as damaged DNA via telomere capping [Links, Images, Papers, Patents, Books].
Statins [Links, Images, Papers, Patents, Books] have been observed to provide an enhancement of the associated telomere protection biology [Paper, Books], but are often avoided due to certain hazards. In certain experiments atorvastatin (0.1 mol/L) and mevastatin (1.0 mol/L) both led to a more than 3-fold increase in the expression of the telomere capping protein TRF2 (telomere repeat-binding factor, Index/Telosome), as shown by immunoblotting. Simvastatin (Zocor) is even more effective in promoting TRF2. Today atorvastatin is available by prescription, but mevastatin is not used due to multiple side effects. Uncapping of telomeres may be detected by the loss of TRF2, and the effect of the statin drugs has been produced by application of exogenous TRF2 [Index/TRF2, Papers, Books], which protects human telomeres from end-to-end fusions. TRF2 binds to double-stranded telomeric DNA. PARP1 interacts with TRF2 and is involved in repairing damaged telomeres. "As the length of telomeres in leukocytes shortened the risk of coronary heart disease increased... the risk was substantially attenuated by pravastatin [Links, Books]. Since statins have been shown to increase the production of a protein-telomere capping protein that prevents telomeres from shortening it is a potential hypothesis that statins bring about some benefits by this mechanism. This is very early research but it implies that statins could well have the ability to slow certain aging processes." - ErinPharm Gazette, Jan. 2007.
I note that my friends have warned me not to meddle with statin drugs due to side effects [article, Books, Links] such as lowering the body's level of CoQ10. Furthermore, work by Woodring E. Wright and Jerry W. Shay showed that TRF2 overexpression can degrade telomeres. Effective treatment to improve levels of telomere-capping associated proteins is, however, definitely on the horizon. Overexpression of TRF2, which stabilizes the telomeric loop, shifts the telomere length threshold for senescence towards shorter telomeres (Karlsreder et al. 2002), so that TRF2 overexpression has been observed to delay senescence. Furthermore, TRF2 overexpression improves double-strand DNA repair (Mao et al. 2007). Recent research shows that TRF2 is a factor in telomere length regulation, and in preventing telomere fusion between chromosomes (Smogorzewska et al. 2000, van Steensel et al. 1998, Celli and de Lange 2005). By 2011, however, there were more indications that TRF2 overexpressionmay be dangerous (M.A.Blasco, 2008, in K. Lenhard Rudolf, 2008, p.283). TRF2 is upregulated in some human carcinomas, along with TRF1 and TIN2 (Oh et al. 2005, Matsutani et al. 2001, Munoz et al. 2005, Nakanishi et al. 2003, Bellon et al. 2006). TRF2 overexpressing mice show increases in chromosomal instability and telomere recombination(Munoz et al. 2005, Blanco et al. 2007) and also rapid and dramatic loss of telomere sequences where skin is exposed to light (Munoz et al. 2005).
Also note that expression of telomerase protects the telomere cap, so that small-molecule telomerase activators such as astragalus extract, astragaloside IV, or cycloastragenol defend telomere capping. Telomere uncapping induces the state of cellular growth arrest termed senescence, or mortality state M1. [Senescence and Immortalization: The Role of Telomeres and Telomerase, Jerry W. Shay and Woodring E. Wright, from Carcinogenesis, 2005]. If the genes maintaining the senescence checkpoint M1 are blocked, say by a viral oncogene, the cells continue to divide and proceed to the crisis state M2, typically followed by cellular apoptosis. Both M1 and M2 can be prevented by introducing telomerase. [Telomere Regulation in Eucaryotic Cells, from Chromosomal Instability and Aging: Basic Science and Clinical Implications By Fuki M. Hisama, Sherman M. Weissman, George M. Martin, Informa Health Care, 2003.] The telomere t-loop closes closer to non-canonical sequences differing from GGTTAG repeat (subtelomeric repeats) as cell divisions proceed and the telomere shortens. The telomeres are reduced by 50-200 bp per division until the telomere tail fails to close into a t-loop(bound with a small d-loop) when TRF2 cannot find a proper binding site due to the non-canonical nature of the repeat there, for instance and typically GGTGAG.
See also Carol W. Greider's Telomeres Do D-Loop T-Loop Minireview, IMGENEX on telomere-associated factors, Jerry Shay's turn-of-the-century article in Nature on on telomeric t-loops and d-loops, Dr. Connie Nugent's lab, and Joao Pedro de Magalhaes on telomeres and telomerase. Recent work shows that telomere-protection protein POT1 is involved in binding the single-stranded overhang at the tip end of the telomere to close the t-loop and is required for telomere length regulation and chromosomal end protection [Yang, et.al, 2007]. Humans have one POT gene, mice have two. Telomeres are bound to a set of numerous interacting proteins [Index] including 6 shelterin subunitsRAP1, TRF1, TRF2, TIN2, TPP1, and POT1, which insure proper maintenance of telomeres [Index]. It has been described as the telomere binding complex [Links, Images Papers, Patents, Books], the telosome [Links, Images, Papers, Books], or more recently shelterin [Links, Images, Papers]. Note that telomeric protein complexes are also being investigated by Dr. Zhou Songyang [Papers, Papers/telomeres and Dr. Zhou Songyang]. Jesse Fender and other investigators including Woodring E. Wright and Jerry W. Shay have been exploring the interaction of the protein Ku and telomerase. Ku (KU70, KU86), which is involved in localizing telomeres to the nuclear membrane, is also important in assisting telomerase function, lack of Ku leading to telomere-telomere fusions and cellular dysfunction. For epigenetic factors in telomere lengthening and corresponding therapeutic possibilities, see Telomeric Chromatin.
Blackburn: Telomerase Does Not Act on Capped Telomeres
Incidentally, according to a model propounded by E. Blackburn in Switching and Signaling at the Telomere (2001), telomerase does not act on capped telomeres, but only on telomeres that have become short enough to become uncapped. Telomere enlongation via telomerase then provides telomeres long enough to cap themselves. This seems to be supported by TA-65 testing in 2010. See Calvin B. Harley, Weimin Liu, Maria Blasco, Elsa Vera, William H. Andrews, Laura A. Briggs, and Joseph M. Raffaele (2010), A Natural Product Telomerase Activator As Part of a Health Maintenance Program, Rejuvenation Research, September 7, 2010. However, telomerase activation can easily result in telomere growth > 55 bp/year. Retinal pigment epithelial cells transfected with hTERT plasmids show telomeres lengthening at 115-255 bp per population doubling, and we find that BJ foreskin fibroblasts similarly transfected show a telomere length increase of 340-370 bp per population doubling, the same order of magnitude (400-460 bp/year telomere growth, corresponding to roughly 1 cell division per year) originally observed at TA Sciences in 2007 by Bob Waskom and Greta Blackburn. - From Andrea G. Bodnar, Michel Ouellette, Maria Frolkis, Shawn E. Holt, Choy-Pik Chiu, Gregg B. Morin, Calvin B. Harley, Jerry W. Shay, Serge Lichtsteiner, and Woodring E. Wright (1998), Extension of Life-Span by Introduction of Telomerase into Normal Human Cells, Science, vol. 279, 16 January 1998. Normally, human fibroblasts can divide for about 49 population doublings.
"Human fibroblasts expressing the catalytic component of human telomerase (hTERT) have been followed for 250–400 population doublings. As expected, telomerase activity declined in long term culture of stable transfectants. Surprisingly, however, clones with average telomere lengths several kilobases shorter than those of senescent parental cells continued to proliferate. Although the longest telomeres shortened, the size of the shortest telomeres was maintained. Cells with subsenescent telomere lengths proliferated for an additional 20 doublings after inhibiting telomerase activity with a dominant-negative hTERT mutant. These results indicate that, under conditions of limiting telomerase activity, cis-acting signals may recruit telomerase to act on the shortest telomeres..." from Michel M. Ouellette, Martha Lia, Brittney-Shea Herbert, Mari Johnson, Shawn E. Holt, Heidi S. Liss, Jerry W. Shay and Woodring E. Wright (2000), Subsenescent Telomere Lengths in Fibroblasts Immortalized by Limiting Amounts of Telomerase, The Journal of Biological Chemistry, April 7, 2000, vol. 275, 10072-10076. See also telomerase artist's conceptions [telomerase & proteins database, telomere image]. Note that telomerase inhibitor RHPS4 [TI/RHPS4, article] acts through stabilization of four-stranded G-quadruplex structures formed by single-stranded telomeric DNA found at the end of the t-loop structure, so that telomerase does not act on the closed telomere loop. I get the impression that the percentage of senescent cells in a tissue determines how old it looks, so that the macroscale human phenotype can be rejuvenated by merely making telomeres long enough to close the t-loops, as far as mitotic cells are concerned.
However, recovery from replicative senescence can be frustrated by high levels of P16INK4A, which is down-regulated by the Id-1 helix-loop-helix transcription factor, itself upregulated by Nerve Growth Factor (NGF), upregulated by acetyl L-carnitine, rosemary tea, and other nutraceuticals. P16INK4A can also be reduced by exercise, carrots, and retinol.
It is certainly true that maintaining long telomeres supports closed-loop telomere structure that frustrates the arrival of replicative senescence, especially in the absence of oxidative stress leading to premature stress-induced senescence. The best way to to keep telomere loops lengthy is probably to use telomerase activators, which have closure possibilities for telomere t-loops to help cells rejuvenate and avoid replicative senescence. However, as cells accumlate more and more P16INK4A, recovery from the state of replicative senescence becomes more difficult. Eventually, perhaps a healthy adult stem cell with vigorously long telomeres can fill a spot where a senescent cell has become apoptotic in such cases. In time, rejuvenated cells secrete collagen, elastin, and endogenous antioxidants in a way that restores a youthful look, although ceroid or lipofuscin deposits may have to be removed with CoQ10, alpha lipoic acid, Piracetam, or other drugs to completely finish the job. Note that collagen and elastin can both be restored in the extracellular matrix by telomerase-activating colostrum skin creams or by telomerase-inhibiting TGF-beta skin creams. There may be milage in unlocking closed telomeric loops for telomerase activation, then resealing them, to achieve even longer telomeres for a still more youthful patterns of gene expression associated with longer telomeres. Can telomeres be made as long as desired by the continued application of telomerase activation? Or does everything stop when the t-loop closes and the cell assumes the immortal phenotype? It is certain that continued application of telomerase activation can supply hundreds of additional cell divisions, and that immortal cells continue to divide in a way that maintains fairly constant or increasing telomere length.
Atorvastatin (Lipitor), Simvastatin (Zocor), TRF2, T-Loop Closure, and Rejuvenation
Theoretically, Lipitor (atorvastatin) should be life-extending via its activation of telomere binding protein TRF2. Applying Lipitor at the M1 state with 4000 remaining base pairs might yield about 4000/50 = 80 further cell divisions and lifetimes on the order of 220 years. The additional cell division capacity should strengthen the immune system. The closure of telomere t-loops by TRF2 should remove senescence effects. It could be that Lipitor (atorvastatin) is more rapidly rejuvenating than anything else we have at just $65/month for the prescription drug. A cheaper version, lovastatin, is available, and Zocor (simvastatin) is more powerful. Also check pravastatin, which should produce similar results. Extra CoQ10 or ubiquinolshould be taken, along with a homocysteine shield and antioxidants, including mitochodrially effective antioxidants like alpha lipoic acid and protective drugs like acetyl L-carnitine. Perhaps the most effective program for antiaging will begin with atorvastatin and end with cycloastragenol/resveratrol cycles. This analysis, however, needs experimental verification and requires more study, including side-effects analysis. Some studies maintain TRF2 overexpression damages telomeres, however, so TRF2 overexpression is unlikely to become a preferred life extension strategy.
Telomere Enlongation with Replication Protein A and hEST1A
Other control proteins associated with telomere maintenance include Replication Protein A and hEST1A. Replication Protein A on human Chromosome 17 [Index, Links, GeneCards/RPA1, (YeastGenome/RPA1, YeastGenome/Telomere Maintenance)], which is present in all eucaryotic cells, has been observed to activate telomerase in yeast. See Vera Schramke, Pierre Luciano, Vanessa Brevet, Sylvine Guillot, Yves Corda, Maria Pia Longhese, Eric Gilson & Vincent Géli, (2003, 2004), RPA regulates telomerase action by providing Est1p access to chromosome ends, Nature Genetics 36, 46 - 54 (2004) Published online: 21 December 2003. Estp1 [YeastGenome/Est1] has been conserved in evolution and is present in humans as hEST1A [Links]. "Overproduction of hEST1A cooperated with hTERT to lengthen telomeres." - See Snow BE, Erdmann N, Cruickshank J, Goldman H, Gill RM, Robinson MO, Harrington L., (2003), Functional conservation of the telomerase protein Est1p in humans, Current Biology 2003 Apr 15;13(8):698-704. hEST1A recruits telomerase holoenzymeby binding to hTERT. (See Diagram). hSMG6 [Links/hSMG6, Links/SMG6] is identical to hEST1A. (See Lingner Joachim, Senior scientist, Telomerase and chromosome end replication, ISREC.) According to Gene Cards, overexpression of SMG6 (in humans hEST1A) results in telomere uncapping. Therefore perhaps hSMG6 or hEST1A can be used to extend t-loop capped telomeres, opening them so that they can be extended by telomerase. Telomerase does not act on telomeres unless the telomere is uncapped, as we saw in the last paragraph. Thus large telomere t-loops corresponding to very youthful cells may be prepared by using hEST1a (hSMG6) together with telomerase, probably by stopping SMG6 towards the end of a treatment period to reseal telomere t-loops. Such cells may have superior staying power, lasting for more cell divisions before uncapping, generating a DNA damage signal, and entering the state of replicative senescence.
"TA-65 alone will reduce the percentage of cells with short telomeres ( < 4 kbp) with minimal effects on mean telomere length..." - Calvin B. Harley, et.al, 2010. Perhaps other telomerase activators will turn out to gradually reduce the percentage of short telomeres ( < 4kb) without modifying average telomere length. To improve on average telomere length much with telomerase activation may require activation of hEST1A and Replication Protein A, so that t-loops can open for telomerase access.
The Telomere DNA Helicases and Co-Factor Magnesium
The WRNp helicase protein (associated with Werner Syndrome) that unwinds DNA turns out to be complexed on the telomere [Image] with other t-loop proteins [Index]. The proper function of WRNp requires Mg2++ as a cofactor, so that telomere maintenance may be improved by taking magnesium supplements. Cells lacking WRN helicase activity show defective telomere lagging strand synthesis [Crabbe, et al, 2004]. Life Extension Magazine has noted that low magnesium levels common in the population lead to high rates of hypertension and sudden death. This is perhaps partially due to premature endothelial senescence linked to WRNp helicase malfunction from Mg2++ cofactor shortfall and associated with high monocyte adhesion and consequent formation of atherosclerotic plaque. The magnesium ion is also an essential cofactor for DNA polymerase whenever human DNA is synthesized without a reverse transciptase like telomerase. This was discovered by Arthur Kornberg in the 1960s. (Magnesium is also a cofactor for RNA polymerases.) Note that magnesium supplementation may be used to decrease high blood pressure in non-senescent cases, lowering the risk of heart attack and stroke.
The Time Machine Effect
[agetransformation.html, lifexnotes2.html/TIME MACHINE]
Cosmic Humor and Pursuit of the Aquarian Soul: Moonwalking Scattered Backwards in Time
By watching Indra became
King of the Gods.
How wonderful to watch.
How foolish to sleep.
- Green and the Great Religions.
Keratinocytes of the epidermis live about as long as the period of the moon by transiently expressing telomerase, showing perhaps 1000 stem cell divisions over a lifetime, and coincidentally the synced-up moon projects the mythos of shedding one's "skin". The book of Proclus on the moon-god's spine is thus a "telomere" at the end of the line on the moon, a haunting "God Particle" or "M.Fossel". Furthermore, the Hayflick and Moorhead revolution of 1961 may be mirrored at the summit of NGC 2264 as our hero makes his way to eternal glory as Orion amid his amazing Winter Crescent flying saucer of stars, shining on the smile of Andromeda in Perseus, the Pleiades, and Aries above like the first light of the Sun on the bottom of the New Moon.
Rejuvenation Rates
Note that TA Sciences, using astragalus extract TA-41 in 2005 on a 3-month-on, 3-month-off cycle for a year measured 460 bp/year telomere growth in blood granulocytes, while aging normally subtracts about 50 bp/year, so that with TA-41 the telomere biotimer moved backwards in time about 9 times as fast as it went forwards when "aging", after normal aging base pair losses were accounted for. That is, we observed a rejuvenation rate of about 9 years per year, or 0.75 years/month. Adults may experience 1 to 2 cell divisions per year, since approximately 50 cell divisions take place from the embryonic stage of development in mitotically active human somatic tissues excepting stem cells and germ cells. Persistence is required in de-aging using telomerase activators, as the process takes place gradually, somewhat like aging itself. However, instead of merely slowing the aging process, telomere remodeling with cyclic telomerase activation is expected to carry its practitioners all the way back to the young adult state, if telomeres measure longer year by year. Bear in mind that antioxidants and antiglycating drugs, DNA repair acceleration, homocysteine screens, cell membrane treatments, and vitamin C should help ensure a successful outcome with a minimum population of senescent cells to threaten cancer or susceptibility to diseases of old age. Note that there are 10 bp/turn in Watson-Crick B-DNA, with 33.2 Angstrom units per turn, or 3.32 nm/turn, so that in one year when 50 bp are lost, we typically lose 16.6 nm of telomere length. On the other hand, using TA-41 according to the Patton Protocol, we should see 9 x 16.6 nm = 149.4 nm of telomere growth per year, amounting to 1494/33.2 = 45 turns of the Watson-Crick DNA double helix at each end of every chromosome, about 76.67 telomeric GGTTAG satellite DNA repeats/year. 149.4 nm is in the EUV extreme ulraviolet range of wavelengths, and more than 2 years of growth are required for the telomere growth length to approach the wavelength of violet light, 380-450 nm. I note TA Sciences TA-41 user Bob Waskom, aged 69, observed a rejuvenation rate of B = By = -8 years per year, based on 400 bp of telomere growth per year, which amounts a to a Bm = - 0.667 years/month rejuvenation scenario. See TA Sciences testamonials.
More recent studies of TA-65 (Calvin B. Harley, et. al., 2011) do not show such dramatic lengthening of telomeres, but rather closing of the shortest telomere t-loop to prevent cellular senescence. It will be useful to measure the rejuvenation rate constant B in years/year for telomerase activators in general, in parallel, in the presence of transcription accelerators, and while on specific diets targeted to improve by reducing the intake or expression of telomerase inhibitors. For instance, it will be useful to test B in connection with schemes for improving the rejuvenation rate such as adding the chromatin-expanding histone deacetylase inhibitor sodium butyrate to improve transcription speeds. Moreover, sodium butyrate greatly enhances levels of the wound-healing telomerase activator epiregulin after exercise by upregulating the transcription factor Sp1. Also, it is expected that testing B (Bikini Atoll... Test B!) will give better results when a low polyphenol diet is used while applying telomerase activators, because high polyphenol diets tend to generate telomerase inhibitors. Now that I have identified endogenous telomerase activators and 15 exercise-induced telomerase activators together with supplements to promote their expression, we have still more measurements to do to define optimal therapy. By February 2013, we have estimated that Product B is associated with a maximum rejuvenation rate of B = -8.218 years/year.
Of all animals, only Leach's Storm Petrel [Wikipedia, Links, Images] has telomeres which lengthen with age. The animal lives from 20 to 35 years and is black with a white mark on the tail resembling a load of salt. I get the impression that it dies of mitochondrial aging preventable in humans by using acetyl L-carnitine with alpha lipoic acid and/or pyrroloquinoline quinone (PQQ). On the other hand, the rainbow trout is a fish with high telomerase levels in all its tissues. I might add that in humans improving hTERT transcription in the nucleus improves DNA protection in mitochondria, a surprising result. hTERT transcription also improves DNA repair in the nucleus generally, although it is most famous for managing DNA repair and regrowth of telomeres.
Tables for the rejuvenation times and costs may be deduced by linear analysis.
Let the final model age tFM be given by tFM = t0 + BΔt, where
t0 is the Initial Actual Chronological Age, and B is the aging rate. Let
B = -8 or -9 years per year aging rate for rejuvenation, so that
tFM = the model age at the end of rejuvenation. In the table below we choose tFM = 25.
Solving for the rejuvenation time Δt, we find Δt = (tFM - t0)/B years.
Then the Actual Age at Final Model Age tFM is t = t0 + Δt, and we have Cost = $76.00(12)(Δt).
Note that B=1 corresponds to normal aging, B > 1 represents accelerated aging,
0 < B < 1 represents decelerated aging, and B < 0 corresponds to rejuvenation.
Rejuvenation Times to Model Age 25 from Initial Age in Years with Cost in Dollars
Initial Actual Chronological Age t010090807060504030Δt-5 Rejuvenation time, B = -5 yrs/yr 15.0.13.0.11.09.07.05.03.01.0Δt-8 Rejuvenation time, B = -8 yrs/yr9.3758.1256.8755.6854.3753.1251.8750.625Δt-9 Rejuvenation time, B = -9 yrs/yr 8.3337.2226.1115.0003.8882.7771.6660.556t = t0 + Δt-5 =
Actual Age-5 at Final Model Age 25115.0 103.0 91.0 79.0 67.0 55.0 43.0 31.0 t = t0 + Δt-8 =
Actual Age-8 at Final Model Age 25109.375 98.125 86.875 75.685 64.375 53.125 41.875 30.625 t = t0 + Δt-9 =
Actual Age-9 at Final Model Age 25108.333 97.272 86.111 75.0 63.888 52.777 41.666 30.556 Cost-5 = $76.00(12)Δt-513,680 11,856 10,032 8,208 6,384 4,560 2,736912Cost-8 = $76.00(12)Δt-88,550 7,410 6,270 5,185 3,990 2,850 1,710570Cost-9 = $76.00(12)Δt-97,597 6,586 5,573 4,560 3,5462,5331,519507
Left: Jeanne Calment, age 25. Right: Jeanne Calment, age 60.
Also see Einstein Greying, Astragalus Extract Program at 2-Year Point, and pics2013may1.html.
"...and bending down beside the glowing bars, murmer a little sadly how love fled,
and paced the mountains overhead,
and hid his face amid a cloud of stars." - When You Are Old, by William Butler Yeats.
Rejuvenation Times from 60 to Final Model Ages tFM in Years with Cost in Dollars
Final Model Age tFM5550454035302520Δt-5 Rejuvenation time, B = -5 yrs/yr 1.02.03.04.05.06.07.08.0Δt-8 Rejuvenation time, B = -8 yrs/yr0.6251.251.8752.53.1253.754.3755.0Δt-9 Rejuvenation time, B = -9 yrs/yr 0.5561.1111.6672.2222.7783.3333.8894.444t = t0 + Δt-5 =
Actual Age-5 at Final Model Age61.062.0 63.0 64.0 65.0 66.0 67.0 68.0 t = t0 + Δt-8 =
Actual Age-8 at Final Model Age60.625 61.25 61.875 62.5 63.125 63.75 64.375 65.0 t = t0 + Δt-9 =
Actual Age-9 at Final Model Age60.556 61.111 61.667 62.222 62.778 63.333 63.889 64.44 Cost-5 = $76.00(12)Δt-5912 1,824 2,736 3,648 4,5605,4726,3847,296Cost-8 = $76.00(12)Δt-8570 1,140 1,710 2,280 2,8503,4203,9904,560Cost-9 = $76.00(12)Δt-9507 1,013 1,520 2,027 2,5343,0403,5474,053
The $76.00/month cost was derived from summing the cost of 3 bottles of Solaray Astragalus Extract (3 x $8.50), 2 bottles of Herb Pharm Astagalus Extract (2 x $11.59), 1 bottle of Solaray Astragalus Root (1 x $7.25), 1/2 bottle per month of Chitosan (0.5 x $10.00), and 1/2 bottle per month of NOW FOODS IGF-1 Liposomal Spray (0.5 x $30.00). This is the basic cost of the telomerase activators used during the 1st 15 days of the two-part month-long treatment cycle. Sometimes I also rub 4 droppers of GAIA Herbs Astragalus Extract in glycerin into my scalp every day during the first 15 days. Recently in 2012, I have been taking 25 grams/day of Now astragalus root powder for the first 15 days plus GAIA astragalus root extract (Green Label, 3 bottles for 15 days), mostly rubbed into the scalp. In addition, during the first part of the cycle I take 3000 mg/day of Vitamin C, 5-10 grams of arginine per day, a Centrum multivitamin pill, 4 x 400 mg Acetyl L-Carnitine with 4 x 200 mg Alpha Lipoic Acid, 2 grams of CoQ10, 4 x Super B-vitamin pills, and 4 x 25 mg DHEA, 4 x 500 mg of NOWcolostrum, 5 x 500 mg of NOW colostrum in 1/6 cup of water for skin cream, 2 grams of ginko biloba, 3 grams of fenugreek seed before bedtime, 4 x 500 mg of black cohosh, and daily exercise. During the 2nd 15-day part of the cycle featuring telomerase inhibitors, I drop the telomerase activators and take telomerase inhibitors including green tea (3 x 60 mg ECGC), curcumin from turmeric mixed with black pepper in water, 4 x 150 mg green tea extract, 4 x 1000 mg omega-3 fish oil, 4 x 200 mg resveratrol red wine complex, and 1 x 300 mcg melatonin, cacao bean products including cocoa and chocolate, and dietary polyphenols, together with the same basic supplements used in the first part of the cycle, 3000 mg/day of Vitamin C, 5 grams of arginine per day, a Centrum multivitamin pill, 4 x 400 mg Acetyl L-Carnitine with 4 x 200 mg Alpha Lipoic Acid, 4 x Super B-vitamin pills, 3 Pomegranate softgels, 4 x 400 IU Vitamin D3, and 4 x 25 mg DHEA. Perhaps I spend $130/month (2007) to $230/month (2013) on supplements, altogether, economizing my obtaining many from discount stores. I also take 2 tablespoons of chocolate powder stirred in water, then heavily iced for antioxidant effect and for extra arginine, several times a day. I believe from toxicology studies that this is safe.
The Pomegranate and the Vitamin D3 supplements help deflect cancer, and Vitamin D3 is an anticancer telomerase inhibitor. Another alternative is Herbal Remedies Astragalus 1.25 mg astragalosides per 250 mg cap, via Nature's Way, ( Standardized 0.5% Astragalosides ), 60 VCapsules per bottle, incuding Astragalus, dried extract 250mg (root) 0.5% astragalosides, with Astragalus (root) 250mg. Four capsules yield 5 mg astragalosides plus 1 gram of astragalus membranaceus root, which improves bioavailability of astragalosides. Obviously, we need more measurements of actual results obtained with off-the-shelf astragalus root ( < 33 grams/day), astragalus extracts, and similar preparations such as TA Sciences TA-65 and RevGeneticsAstragaloside IV or cycloastragenol Astral Fruit-C to verify tables like this table for rejuvenation to effective telomeric chromosomal age 25. The pioneering research in the matter was done by Geron [Geron Patent, A', A''], TA Sciences, Telomolecular Nanotechnologies, VIDA Institute, Terraternal, RevGenetics, and other firms including Sierra Sciences. They are still trying to develop faster, more effective telomerase activators that get results quicker. Measurements of telomere growth in base pairs may be done through Repeat Diagnostics for less than $700 per pass as suggested by RevGenetics, via TA Sciences if one is enrolled in their program, via Spectracell Laboratory, via Life Length, Telome Health, or via kits and software described in our labs section. See the rejuvenation tables for my self-experiment in anti-aging therapy with astragalosides at agetransformation.html, and my video Anti-Aging Therapy with Astragalosides.
Note that decelerated aging with 0 < B < 1 corresponds to the usual program of anti-aging medicine without telomerase activation, a footdragging delay in the aging process. For instance, vitamin C or homocysteine blockers decelerate the aging process, slowing the removal of telomere base pairs from chromosome tip ends. On the other hand, the aging regimes with B < 0 correspond to rejuvenation with telomerase activation. Plotting the final model age tFM = t0 + BΔt as a function of t = t0+ Δt yields charts of aging as controlled by stepwise modification of the aging rate B(t).
Let Δt be the treatment time for a desired age transformation from a certain Chronological Age to a younger Desired Model Age. Then
(Chronological Age - Desired Model Age)/9 < Δt < (Chronological Age - Desired Model Age)/8,
where the ages are given in years and 8 and 9 years per year are the limiting absolute B-factor magnitudes for the age transformation rates.
Deaging Plot: See the Model Age spread at the 58th, 60th, 61st, 62nd, 63rd, 64th, 65th birthdays.
Based on TA Sciences 8 years per year to 9 years per year rejuvenation rate results obtained for TA-65 at 5 mg/day using the Patton Protocol. B = - 5.2 years per year was initially estimated for astragalus extract, and B = - 5.18684 shows that this was close enough for the data quality available. All model ages in the above drawing correspond to May Day, as treatment started on May 1, 2007, whereas my birthday is on May 13, 1949. After 2.5 years at about 60.5 when bone dry I seemed to be 45 to myself, whereas with oiled hair I seemed to restaurant help to be 40 at 60. This led to the B = - 5.2 years per year estimate for the astragalus extract rejuvenation rate.
By 2014, however, I note that with astragalus NK cells rejuvenate much faster than other cell types. With exercise and HGH, deaging can be more uniform, as all human cells have about the same density of GHR, the HGH receptor, in their cell membranes.
Deaging Plot Corresponding to Astragalus Extract Observations after about 2.5 years.
(From 58 to Model Age 45 at 60.5 in 2.5 years, projected to Model Age 25 at 64 in 6.33 years.)
The observed rejuvenation rate is computed at B = -5.18684 years per year, approximately B = -5.2, corresponding to the top line on the chart. At the present time, I may seem to be 45, so that at the B = -5.18684 rate I would achieve a 25 year old appearance at about 64.33 years of age in 2013. The dotted lines are for TA-65 at -8 and -9 years per year. Basic cost is about $55/month, including Chitosan, or $660/year, yielding $4,188 for 6.34 years to 25 at 64. Extra money might be spent on CoQ10, alpha lipoic acid with acetyl L-carnitine, magnesium, zinc, vitamin D, multiple vitamins, resveratrol for the off part of the cycle, and other refinements.
Anti-Aging Flight Plan (reminiscent of My World Line by George Gamow). 64.3 = August 31, 2013. Δt = 6.3 years.
Here Model_Age = Bt + Starting_Age, B=1 for normal aging, B > 1 for accelerated aging, B=0 for no aging, and B < 0 for rejuvenation, or negative aging. Here initially B= 1 for normal aging, then
B= - 5.2 years/year during the deaging phase, followed by B=0 zero aging until Madame Jean Louise Calment's 122 years, 164 days record is exceeded at 2071.9634 AD, which should be right at the Winter Solstice when shadows are shortest about Dec 22, 2071. About this time the S.Monocerotis skywalker above the Cone Nebula crosses the meridian. Note the "Cross of Years" formed by the 110 and 122.45 barriers. There are thousands of Americans over 100, of whom hardly any will get though the 110 year barrier without medicine. See the associated response of the cloud cover to the inclusion of these charts including the sign of the cross in Visionary Sky 46.5. Note that the B = 0 line in the flight plan can be implemented by using astragalus extract one month out of six, which would lead to a barely discernable jaggle on the B=0 line between 25 and 25 + 5/12 = 25.42 with a five-month rise time and a one-month fall time, since (1/12)(-5.2) = -0.43.
Phase I: For 0 < t < 57.967123 = t0, B = 1, Model Age = Bt. Ended May 1, 2007.
Phase II: For 57.967123 < t < 64.3, B = - 5.2 years/yr,
Model_Age = B(delta_t) + t0 = (B/12)N + 57.967123,
N = 1, 2, 3,...,76 months, using off-the-shelf astragalus extracts. Started May 1, 2007. See Quick Summary for details.
Phase III: For 64.3 < t < infinity, B = 0, Model Age = 25. Starts at August 31, 2013.
Note that the Flight Plan chart may be similar if the observed approximate B = -5 years per year rejuvenation rate is due to a reduction in the number of short telomere ( < 4 kbp) cells, rather than to an increase in average telomere length. That this may be true is indicated by recent research on TA-65. (Calvin B. Harley, et.al, 2010). Average telomere length growth may require hEST1A and Replication Protein A to open telomere t-loops when the length is greater than 4 kbp, or phosphorylation of tankyrase 1 with insulin from Fenugreek seeds to open telomere loops by stripping telomere loop closure protein TRF1.
On the other hand, Retinal pigment epithelial cells transfected with hTERT plasmids show telomeres lengthening at 115-255 bp per population doubling, and we find that BJ foreskin fibroblasts similarly transfected show a telomere length increase of 340-370 bp per population doubling, the same order of magnitude (400-460 bp/year telomere growth, corresponding to roughly 1 cell division per year) originally observed at TA Sciences in 2007 by Bob Waskom and Greta Blackburn. - From Andrea G. Bodnar, Michel Ouellette, Maria Frolkis, Shawn E. Holt, Choy-Pik Chiu, Gregg B. Morin, Calvin B. Harley, Jerry W. Shay, Serge Lichtsteiner, and Woodring E. Wright (1998), Extension of Life-Span by Introduction of Telomerase into Normal Human Cells, Science, vol. 279, 16 January 1998.
Estimated Rejuvenation Times = T to Target Model Age t1
from Starting Age t0 for B= -5.2 years/year
with Chronological Age After Treatment = t0 + T.
Treatment Time T(t0, t1) = (t0 - t1)/5.2 years, T(ray, target) = Treatment Time Chronological Age after Treatment = t0 + T = t0 + [(t0 - t1)/5.2] years = Final Age. Treatment Time (yr), Age after Treatment | Treatment Time (yr), Age after Treatment (yr) Start Target Duration Target Final Age | Start Target Duration Target Final Age t0 t1 T t1 t0 + T | t0 t1 T t1 t0 + T T(110, 25) = 16.346, Age(25) = 126.346; T(105, 25) = 15.385, Age(25) = 120.385, T(100, 25) = 14.423, Age(25) = 114.423; T(95, 25) = 13.462, Age(25) = 108.462, T(90, 25) = 12.500, Age(25) = 102.500; T(85, 25) = 11.538, Age(25) = 96.538, T(80, 25) = 10.577, Age(25) = 90.577; T(75, 25) = 9.615, Age(25) = 84.615, T(70, 25) = 8.654, Age(25) = 78.654; T(65, 25) = 7.692, Age(25) = 72.692, T(60, 25) = 6.731, Age(25) = 66.731; T(55, 25) = 5.769, Age(25) = 60.769, T(50, 25) = 4.808, Age(25) = 54.808; T(45, 25) = 3.846, Age(25) = 48.846, T(40, 25) = 2.885, Age(25) = 42.885; T(35, 25) = 1.923, Age(25) = 36.923, T(110, 35) = 14.423, Age(35) = 124.423; T(105, 35) = 13.462, Age(35) = 118.462, T(100, 35) = 12.500, Age(35) = 112.500; T(95, 35) = 11.538, Age(35) = 106.538, T(90, 35) = 10.577, Age(35) = 100.577; T(85, 35) = 9.615, Age(35) = 94.615, T(80, 35) = 8.654, Age(35) = 88.654; T(75, 35) = 7.692, Age(35) = 82.692, T(70, 35) = 6.731, Age(35) = 76.731; T(65, 35) = 5.769, Age(35) = 70.769, T(60, 35) = 4.808, Age(35) = 64.808; T(55, 35) = 3.805, Age(35) = 58.805, T(50, 35) = 2.885, Age(35) = 52.885; T(45, 35) = 1.923, Age(35) = 46.923, T(40, 35) = 0.962, Age(35) = 40.962.
I think perhaps these effects can be reliably mobilized for $130 x 12= $1560/year, so for each column of treatment time T,
Approximate Cost = $130 x 12 x T = $1560 x T = $9,828.00 after 6.3 years
Thus we may now have anti-aging medicine and associated technique to survive hundreds of years, although the medicine acts very slowly, like aging itself, to make us young again. Each month the expense of it satisfies
Cost/month > $130, perhaps $130 < Cost/month < $150,
depending on how thoroughly we treat every variable. Of course, still more can be spent by very careful experimenters, especially if they cautiously employ blood testing to measure their age variables and telomere lengths. By the 22nd century, the merit of telomerase activation in connection with anti-aging treatment will be obvious, but for decades to come until decisive demonstrations are readily available from many experimenters, time and expense may veil the solution. A more effective, safe enough, fast-acting anti-aging telomerase activation technique could save many lives merely by subtracting the elements of expense, long suffering, and mystery.
Fast-Acting Techniques
So far, one email correspondent has suggested that 9.5 years per year rejuvenation results have been observed with schemes like ours, and up to 460 bp/year of telomere growth has been observed with the cyclic Patton Protocol at TA Sciences using the astragalus extract TA-41 corresponding to absolute rejuvenation rates of up to 9 years per year. Subsequent observations (Calvin B. Harley, et.al, 2011) suggest that TA-65 merely closes the shortest telomere in a cell to prevent replicative senescence. Fast rejuvenation rates may be possible with adequate safety. Very high speeds have been reported using nucleoside-modified hTERT mRNA. We need to measure the telomere growth rates for the safer of the 178 various telomerase activators we have listed at varying concentrations in order to gain more penetrating insight. Also, mixtures of the telomerase activators [Index] combined for parallel activation pathways may yield superior results in the future. Better methods for improving the bioavailability of the telomerase activators used may improve the cost/benefit ratio. I note that dogs and rats can tolerate higher doses of astragalus extracts than we presently use. See Shu-Yi Yua, Hong-Tao OuYanga, Ju-Yun Yanga, Xiao-Liang Huanga, Ting Yanga, Ju-Ping Duana, Jun-Ping Chenga, Yu-Xiang Chen, Yong-Jia Yanga and Pang Qiong (2007), Subchronic toxicity studies of Radix Astragali extract in rats and dogs, Journal of Ethnopharmacology, Volume 110, Issue 2, 21 March 2007, Pages 352-355. "In conclusion, our studies clearly demonstrated that Radix Astragali Extract [Images] was safe without any distinct toxicity and side effects, the safety dosage range is 5.7–39.9 g/kg for rats and 2.85–19.95 g/kg for beagle dogs, which is equal to 70 or 35 times of that of human (0.57 g/kg, say, average BW 70 kg), respectively."
See also Astragalus Extract toxicity. Thus to rejuvenate more rapidly, it may be useful to take more astragalus extract in divided doses throughout the day. In addition, we may use chitosan (perhaps 2 grams/dose) to improve the bioavailability of astragalosides. Better measurements of astragaloside bioavailability improvements with varying doses of chitosan would be helpful, and perhaps Internet searches can help us locate more existing data. Perhaps we can use a chromatin-expanding HDAC inhibitor such as sulforaphane from broccoli sprouts, diallyl sulphide from oysters or garlic, CGK 1026, Tricostatin A, sodium butyrate [Index] or the HDAC inhibitor sodium 4-phenylbutyrate [Index], to accelerate transcription of hTERT mRNA, although this is now only solidly established for CGK 1026 and Tricostatin A. We may use a diet emphasizing low-polyphenol foods during periods in which astragalus extract is taken, in order to steer clear of telomerase inhibitors generated by high-polyphenol foods. On the other hand, short duration of the effects such telomerase inhibitors may make this unnecessary. Otherwise, we may take medicines or growth factors such as IGF-1 that phosphorylate hTERT with Akt, tending to import hTERT protein from the cytosol into the nucleus. To this we can add Fenugreek extract with < 3 grams niacinamide for NAD+ substrate synthesis, to open telomere loops with Fenugreek's 4-hydroxyisoleucine insulin stimulation, phosphorylating tankyrase 1 to remove closure protein TRF1 with poly(ADP-ribo)sylation, making telomere tips accessible to the telomerase holoenzyme, and simultaneously stimulate hTERT mRNA transcription with HIF-1 transcription factor promoted by Fenugreek's diosgenin. Note that Ginkgo Biloba also stimulates production of HIF-1. Furthermore, we may use antioxidants such as glutathione and N-acetylcysteine to retain hTERT inside the nucleus [Papers]. Parallel path activation of hTERT transcription using HGH from HGH secretagogues may also help accelerate hTERT mRNA transcription without causing unacceptable transformations due to gene mutation, gene translocation, or gene amplification that can lead to cancer.
Recovery from Cellular Senescence
I note that recovery from advanced cellular senescence may be more difficult than merely defending ourselves against the arrival of replicative senescence in the first place. Patients should probably be encouraged to start early, at the fist sign of a grey hair, to conserve their sex appeal and fortify them against atherosclerosis, cancer, and other diseases of old age [charts]. Our insight into what may be achieved with small molecule telomerase activators [Index] and supporting therapies will change as more and more very aged patients attempt rejuvenation. Ultimately, it may be useful or necessary to take steps such as inserting extra genes for antisense mRNA to oppose further accumulation of factors supporting halt of the cell cycle like P16INK4A or p21Waf1/Cip1 using Zinc Finger Nuclease technology, for instance. However, until we know whether or how fast these are consumed by ubiquitination, we will probably rely on activating hTERT mRNA transcription or inserting the hTERT gene. An extra gene for hTERT might be inserted at the 25th year, administered like an oral liposomal spray or if need be like a flu shot. However, we are approaching final victory over death from degenerative diseases associated with aging (and otherwise) as controlling factors from molecular biology come more clearly into focus and our medical bioscience techniques evolve to meet the challenges. Perhaps medicines, diet, and exercise alone will usally be adequate to achieve physical immortality without modifying the human genome.
Therapy for recovering from cellular senescence [Restoring Senescent Cells | Refs 9 | Refs 10]
Telomerase activators allow us to restore youthful patterns of gene expression prior to cellular senescence, preventing replicative senescence; we also fortify the cell against stress-induced senescence by using antioxidants to protect telomeres against ROS. Michael Fossel [Fossel, (1998), JAMA] noted that telomerase can rejuvenate senescent cells. Sierra Sciencespoints to rejuvenated skin experiments: Walter D. Funk, C.Kathy Wang, Dawne N. Shelton, Calvin B. Harley, Garrett D. Pagon, Warren K. Hoeffler (2000), Telomerase Expression Restores Dermal Integrity to in Vitro-Aged Fibroblasts in a Reconstituted Skin Model, Experimental Cell Research, 2000. "Telomerase activity not only confers replicative immortality to skin fibroblasts, but can also prevent or reverse the loss of biological function seen in senescent cell populations." However, after cells become senescent, telomerase activators are sometimes incapable of rescuing the cell, and cells experience growth arrest due to cell membrane communications failure from high caveolin levels in which the cells are not responsive to growth factors such as EGF and PDGF, and they also experience the change to the larger senescent cellular phenotype, in which they resist external stimuli and apoptosis, a survivalist response of the aging cell.
This seems to be partly due to a pinch-off of cell membrane caveolae with rising caveolin-1 levels and their subsequent internalization as detached vesicles, stopping signaling through open flask-shaped caveolae on the cell membrane in which key signaling receptors are located. Thus caveolin-1 behaves as a gatekeeper molecule for terminating signaling functions associated with endocytosis, shutting down certain membrane communications associated with sensitivity to growth factors such as EGFand PDGF as caveolin-1 levels rise, producing growth arrest. Rising caveolin-1 levels also increase p53 and p21 expression, which acts to stop the cell cycle, mediating cell cycle arrest through a p53/p21Waf1-dependent pathway. Reducing caveolin-1 status decreases p53 and p21 expression, enabling recovery from the senescent state and restart of the cell cycle by telomerase-activating growth factors. Senescent cells may be rejuvenated by sequestering FOXO transcription factors away from the nucleus to down-regulate caveolin-1 expression. This makes senescent cells once again responsive to human growth factor telomerase activators such as EGF or PDGF, cures the sluggish hyporeactivity of senescent cells, and restores the youthful cellular phenotype. After lowering caveolin-1 the cell cycle can be restarted with DNA synthesis and lowered levels of cell cycle inhibitors p53 and p21 by stimulation with EGF or PDGF, both components of colostrum. (KA Cho, SJ Ryu, JS Park, IS Jang, JS Ahn, KT Kim, and SC Park, 2003). "Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation..."
Note that folic acid (List, vitamin B9) stimulates the PI3K/AKT pathway to down-regulate caveolin-1 by sequestering FOXO factors away from the nucleus. Thus a large dose of folic acid (1 to 5 mg, avoid cramps) along with a bodybuilding workout set up to elevate EGF and PDGF with exercise and supplements such as colostrum might return a man's senescent cells rapidly to a rejuvenated state. Exercise-intensive treatment is an experiment for patients without atherosclerotic plaque, arteriosclerosis, or aortic stenosis, however.
Skin Creams for Rejuvenating Senescent Dermal Fibroblasts [Expanded Notes]
A rejuvenating skin cream might be prepared with telomerase-activating colostrum (containing EGF and PDGF) mixed with folic acid (to inhibit caveolin-1, gene CAV1) to implement restoration of senescent dermal fibroblasts. It is useful to mix it in green tea. Note that colostrum also contains VEGF, which upregulates survivin to restore senescent cells. This may be combined with treatment to simultaneously elevate cyclic AMP (with glycyrrhiza extract, exercise, or forskolin), which also downregulates caveolin-1. Magnesium and alpha lipoic acid may be added to supply required cofactors including HSP90 to support telomerase assembly. Treatment with retinol [which converts into retinoic acid (List)] might be added to this to inhibit P16INK4A, which makes recovery from the senescent state difficult. However, retinol [List] should be applied during the two-week telomerase inhibiting part of the cycle of telomerase activation followed by telomerase inhibition.
Small molecule caveolin-1 inhibitors such as N-[2-(Cyclohexy-Loxyl)-4-Nitrophenyl]-Methanesulfonamide and forskolin's cAMP may be the most effective solution, because the senescent state of the cell causes it to somewhat resist insulin stimulation and other stimulation from large molecules such as IGF-1 interacting with membrane receptors. Still, downregulating caveolin-1 with insulin or IGF-1 has at least preventative value in resisting the onset of senescence. Insulinor IGF-1 (which influences every cell in the body) can be used to downregulate FOXO and thus downregulate caveolin-1prior to the onset of senescence. Insulin-boosters include:
(1) Alpha Lipoic Acid taken 600-1000 mg after a workout can increase fat-burning and insulin-stimulated glucose uptake.
(2) Banaba Leaf Extract (taken at 32-48 mg with a postworkout shake) improves insulin sensitivity.
(3) Gymnema Sylvestre (400-500 mg with a postworkout shake < 30 min after exercise) stimulates insulin secretion.
(4) Fenugreek seed or Fenugreek Extract [Images] stimulates insulin production via
(5) 4-hydroxyisoleucine [Images] isolated from Fenugreek seeds.
Dextrose may be taken at 25-50 grams to spike insulin, along with whey protein or whey hydrolysates after a bodybuilding workout.
IGF-1 may be produced in the liver from HGH (Telomerase Activators/HGH) stemming from, say, whey protein, exercise, or HGH secretagogues such as alpha-GPC. Casein (cottage cheese) elevates IGF-1 levels, as does orally ingested colostrum[List]. Acetyl L-carnitine restores levels of IGF-1 in aging neural tissue. The growth-promoting effect of IGF-1 on brain cells is potentiated by acetyl L-carnitine plus alpha lipoic acid. Note that creatine monohydrate also stimulates the production of IGF-1, which activates the PI3K/Akt pathway, excluding FOXO from the nucleus to down-regulate caveolin-1(gene CAV1) expression and prevent cellular senescence. However, senescent cell membrane caveolae are required to communicate with IGF-1, but don't exist when caveolin-1 is too highly expressed, so that IGF-1 is more useful for preventing senescence than for reversing it. Bodybuilding exercise with creatine monohydrate suitably elevates IGF-1 to inhibit FOXO transcription factors via the IGF1/PI3K/Akt pathway, thus inhibiting caveolin-1 transcription to prevent senescence. Note that the "PI3K–Akt pathway is a major upstream signaling module leading to the phosphorylation of FOXO factors, their exclusion from the nucleus," and subsequent ubiquitination by proteasomes. - from (Dominique A Glauser and Werner Schlegel (2007)).
Treatment should be applied on a cyclic basis with telomerase inhibitors to guard against cancer. Other telomerase activators may be used on a cyclic basis with telomerase inhibitors to lengthen telomeres while striving (with colostrum and caveolin-1 inhibitors to overcome the sluggish hyporeactivity of the senescent cell membrane associated with overexpression of caveolin-1 due to FOXO transcription factor overexpression in senescence and to regain youthful cell morphology while restarting the cell cycle and rejuvenating the senescent cell. Note that caveolin-1 is up-regulated by low density lipoprotein free cholesterol, so that high free cholesterol levels could lead to cellular senescence. This may be controlled with pantethine (vitamin B5), which lowers LDL cholesterol. (van den Heuvel, Schulze, and Burgering, 2005). See also Bist A., Fielding P. E., Fielding C. J. (1997), Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol, Proc. Natl. Acad. Sci. U.S.A. 1997;94:10693–10698. This article defines the caveolin-1 promoter. Note that when senescent cells are rejuvenated by downregulation of caveolin-1, the restored cell not only recovers its youthful phenotype and sensitivity to growth factors, but also lengthens its telomeres quite substantially to a halfway point allowing typically 25 further cell divisions. Further improvements in telomere length and replicative capacity can be obtained by applying suitable telomerase activators. Unfortunately, sometimes related simple schemes can be upset by low levels of vitamin K2 resulting in aortic stenosis calcification which forbids regular hard exercise.
Caveolin-1 is downregulated by c-Myc
It turns out that c-Myc downregulates caveolin-1 expression, and that c-Myc can be upregulated by EGF, PDGF, colostrum, or more dangerously by estradiol [Ref, (Tsai L.-C., Hung M.W., et al, (1997))]. PDGF is upregulated 1.55 times by 30 minutes of exercise. Note that estradiol increases stroke incidence and should be kept within 20 to 30 pg/ml in blood samples.
References:
See Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, Park SC (2003), Senescent phenotype can be reversed by reduction of caveolin status [Papers], Journal of Biological Chemistry, 2003 Jul 25;278(30):27789-95, and also Park SC, Cho KA, Jang IS, Kim KT, Ryu SJ.(2004), Functional efficiency of the senescent cells: replace or restore? [Papers, Full Text pdf], Annals of the New York Academy of Sciences, 2004 Jun;1019:309-16. Also see A. Pieter J. van den Heuvel, Almut Schulze, and Boudewijn M. T. Burgering (2005), Direct control of caveolin-1 expression by FOXO transcription factors, Biochem J. 2005 February 1; 385(Pt 3): 795–802. See also Dominique A Glauser and Werner Schlegel (2007), The emerging role of FOXO transcription factors in pancreatic b cells [Papers], Journal of Endocrinology (2007) 193, 195–207. See also Brain P. Ceresa and Sandra L. Schmid (2000), Regulation of signal transduction by endocytosis [Papers], Current Opinion in Cell Biology 2000, 12:204–210, which explains the role of caveolae in signal transduction.
Survivin and Cellular Senescence Recovery
Note that cellular senescence may also be recovered from by upregulating survivin. Survivin is upregulated by VEGF and bFGF (FGF2), both of which are contained in colostrum [List], which coincidentally contains insulin and improves expression of IGF-1 when taken orally. Note that VEGF is upregulated 1.36 times by 30 minutes of exercise. "Senescence is a reversible process controlled by survivin: by overexpressing survivin in senescent cells, we are able to decrease senescent markers and increase cell proliferation." (Caterina A. M. La Porta, Stefano Zapperi, James P. Sethna (2012), Senescent Cells in Growing Tumors: Population Dynamics and Cancer Stem Cells, January 2012 Issue of PLoS Computational Biology). Survivin should be upregulated on a cycled basis with telomerase inhibitors to guard against cancer.
N-[2-(Cyclohexy-Loxyl)-4-Nitrophenyl]-Methanesulfonamide (NS-398) for Recovering from Senescence
A selective COX-2 inhibitor inhibits senescence in one case, and decreases expression of caveolin-1 in the cell membranes of senescent cells, enabling recovery from cellular senescence when the cell is stimulated with telomerase-activating growth factors such as EGF or PDGF. The use of selective Cox-2 inhibitor N-[2-(Cyclohexy-Loxyl)-4-Nitrophenyl]-Methanesulfonamide [NS-398, 3D, Toxicity] has been patented for this application by S.C.Park and J.A.Han (2008-2012). This substance is believed to transcriptionally inhibit caveolin-1 expression. Side effects are still in question, although the selective COX-2 inhibitors lack the side effects of conventional NSAIDS. See Restoring Senescent Cells, Therapy for Recovering from Cellular Senescence, and small molecule caveolin-1 inhibitors [Papers, Patents].
Forskolin down-regulates levels of caveolin-1 via cyclic AMP to Rejuvenate Senescent Cells
Forskolin is the active ingredient in coleus forskohlii [Images, Video, Papers, Books], which activates the enzyme adenylate cyclase, which converts ATP to cyclic adenosine monoposphate (cAMP). Creatine monohydrate produces more ATP for forskolin-activated adenyl cyclase to convert to cAMP. Epinephrine also acts to stimulate adenyl cyclase to produce cAMPfrom ATP; see epinephrine supplements and epinephrine boosters, such as coffee caffeine, which inhibits cAMP phosphodiesterase, the enzyme that metabolizes cyclic AMP. Two amino acids phenylalanine and tyrosine, sold as supplements, are converted into dopamine. Dopamine, in turn, is converted into norepinephrine, and then epinephrine, which produces more cAMP from ATP. (after Ray Sahelian on Neurotransmitters.) Cyclic AMP (a second messenger) acts by activating protein kinase A (cAMP-dependent protein kinase), which can phosphorylate specific proteins that bind to promoter regions of DNA, causing increased expression of specific genes, or phosphorylate proteins that may act directly on a cell's ion channels, or phosphorylate proteins that may become activated or inhibited enzymes. "It is known that forskolinvia cAMP can down-regulate mRNA levels (of caveolin-1) in a dose-dependent manner.". - after A. Pieter J. van den Heuvel, Almut Schulze, and Boudewijn M. T. Burgering (2005), Direct control of caveolin-1 expression by FOXO transcription factors [Papers, Patents, Books] Biochem J. 2005 February 1; 385(Pt 3): 795–802. The "senescent phenotype, however, can be reversed by reducing the expression levels of caveolin-1, suggesting that either the senescent state is a reversible phenotype or that these cells are actually quiescent, rather than senescent.". See upregulating cAMP and supplements for upregulating cAMP. Schizandra berry is known to increase cyclic AMP in the liver, and licorice (Glycyrrhiza extract) also promotes cyclic AMP and inhibits cAMP phosphodiesterase, which degrades cAMP. "The effect of one bout of intense swimming caused significant increases in the cyclic AMP content of fast-twitch white skeletal muscle, liver, and heart. Further investigation of the exercise-induced increase in myocardial cyclic AMP indicates that the nucleotide content remained elevated long after (24 h) termination of exercise. This increase in cyclic AMP was time dependent, with the level increasing gradually throughout the work bout.
The increase in cardiac cyclic AMP seemed to be independent of work intensity, provided that work time was of sufficient duration (greater than or equal to 30 min)." - After Palmer WK (1988), Effect of exercise on cardiac cyclic AMP, Med Sci Sports Exerc 1988 Dec;20(6):525-30. Note that 2 ligands of the EGF receptor, epiregulin and amphiregulin, are elevated by exercise, as is PDGF, so that exercise reduces caveolin-1 levels with cyclic AMP, restoring senescent cell communications through the cell membrane, then restores senescent cells with longer telomeres via EGF family growth factors (See also EGF), PDGF, and other telomerase activators from exercise. Negative effects of high cAMP may include high body heat associated with thermogenesis and weight loss, estrogenic side-effects from stimulation of aromatase activity, and depression if dopamine levels are not kept up. Again, forskolin therapy may be combined with cyclic telomerase activation to induce telomere growth to restore and rejuvenate senescent cells. See also Yamamoto M., Okumura S., Oka N., Schwencke C., Ishikawa Y. (1999), Downregulation of caveolin expression by cAMP signal, Life Sci. 1999;64:1349–1357. Note, however, that in some cell types cAMP induces cell cycle arrest. (van den Heuvel, Schulze, and Burgering, 2005). For instance, forskolin inhibits colon cancer cell growth and survival (Wikipedia/Forskolin), and forskolin may also suppress leukemia progression (LEF, Nov 15, 2005).
Experimental siRNA restoration of Senescent Cells
These experiments down-regulated caveolin-1 using siRNA, targeting 5 regions of caveolin-1 mRNA, using 21-nucleotide siRNAs obtained from Dharmacon Research (Lafayette, Co). Caveolin-1 was also downregulated with caveolin-1 antisense oligonucleotide (TTTGCCCCCAGACAT) transfected with Lipofectamine. After lowering caveolin-1 the cell cycle can be restarted with DNA synthesis and lowered levels of p53 and p21 by stimulation with EGF (a component of colostrum). (KA Cho, SJ Ryu, JS Park, IS Jang, JS Ahn, KT Kim, and SC Park, 2003). "Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation..." Small interfering RNA oligonucleotides directed against caveolin-1 have included siRNA described in (van den Heuvel, Schulze, and Burgering, 2005).
Arginylglycylaspartic acid for Senescent Cell Morphology Restoration
Most recently treatment with the tripeptide Arg-Gly-Asp (RGD, or Arginylglycylaspartic acid [Links, Images, Papers, Patents, Books; 3D; Toxicity]) has transformed senescent human fibroblasts back to their "young-like phenotype" morphology in 5 days. H. Choi, J.H. Rhim, S.J. Lee, K.A. Cho, S.C. Park (2012), Induction of morphological restoration of senescent, Sens Foundation, 2012.
SIRT1 Deacetylates and activates Ku when Young Extracellular Matrix rejuvenates Senescent Cells
Young extracellular matrix can rejuvenate senescent fibroblasts. During the process, SIRT1 influences DNA repair and deacetylates and activates the chromosomal fusion protection and telomere protection heterodimer Ku composed of Ku70and Ku80 (Ku86). Thus SIRT1-boosters like caloric restriction and resveratrol seem quite promising in connection with recovery from the senescent state of the cell. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix, Aging Cell, 2011 Feb;10(1):148-57. See the related patent by Sang Chul Park, Kyung A Cho, Moon Kyung Ha and Hae Ri Choi, (2011 & 2009), Senescence Control Composition Containing Extracellular Matrix Components[Links, Papers], US20110150899.pdf (2011).
Tocotrienol-rich fraction can reverse senescence in human diploid fibroblasts
Gamma tocotrienol [List] reduces expression of collagenase from senescent fibroblasts, protecting the extracellular matrix, and has favorable impact on gene expression in both senescent and normal fibroblasts, tending to oppose aging. Tocotrienol-rich fraction containing alpha-tocopherol and tocotrienols alpha, beta, gamma, and delta from rice bran, palm oil, oat, or barley, tends to produce telomerase activity in senescent fibroblasts, lengthens telomeres, restores fibroblast morphology, and restarts the cell cycle. [Images/Tocotrienol-rich fraction supplements with alpha-tocopherol from palm oil; Images/Tocotrienol-rich fraction skin cream with alpha-tocopherol from palm oil]. See Suzana Makpol, Azalina Zainuddin, Kien Hui Chua, Yasmin Anum Mohd Yusof, and Wan Zurinah Wan Ngah (2012), Gamma-tocotrienol modulation of senescence-associated gene expression prevents cellular aging in human diploid fibroblasts [NCBI DOC], Clinics (Sao Paulo) 2012 February; 67(2): 135–143, and also Makpol S, Durani LW, Chua KH, Mohd Yusof YA, Ngah WZ (2011), Tocotrienol-rich fraction prevents cell cycle arrest and elongates telomere length in senescent human diploid fibroblasts, [NCBI DOC], Journal of Biomedicine and Biotechnology 2011 Mar 30. Gamma tocotrienol has increased animal lifetimes by up to 18%.
Geron
Midnight Blue: Astragalus Extract to the Scalp Darkens Hair in About 12 Months
Jim Green - Left: Jan 24, 2010, Age: 60.7. Right: Jan 18, 2011, Age: 61.68, Math Model Age: 38.71.
After about a year of astragalosides to the scalp, hair color improved as telomere cell DNA was repaired, activating quiescent hair follicle stem cells in the hair follicle bulge region, initiating cycles of proliferation and hair synthesis.Human dermal fibroblasts can divide about 50 times in culture, as Hayflick & Moorhead discovered in 1961. But with telomerase enzyme enough, the cells become immortal, and have divided hundreds of times in culture. I took TerraternalAstragaloside IV 100 mg/day during the activation part of the cycle during the last few months. GAIA Herbs astragalus root extract in glycerin at 1 mg astragalosides per 30 drops was used for the scalp. HGH to promote hTERT mRNA transcription was boosted by 1200 mg/day Alpha GPC plus exercise. I decided it would be best to include direct topical application, as Artandi Labs at Stanford University reported positive hair restoration results on experimental animals with direct application of astragalosides to hairy skin. "Here we show that conditional transgenic induction of TERT in mouse skin epithelium causes a rapid transition from telogen (the resting phase of the hair follicle cycle) to anagen (the active phase), thereby facilitating robust hair growth. TERT overexpression promotes this developmental transition by causing proliferation of quiescent, multipotent stem cells in the hair follicle bulge region." - after Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE (2005), Conditional telomerase induction causes proliferation of hair follicle stem cells, Nature, 2005 Aug 18;436(7053):1048-52. Most recently (August 2011) I have tried using an ethanolic extract of Fenugreek seed as a telomerase activator [List] on my scalp, which is relatively inexpensive. Colostrumsolution was also under test for treating hair greying. Nothing worked well except application of green flag GAIA Herbs astragalus extract in alcohol or glycerin to the scalp, which takes about a year to modify hair color from the roots out. Further Remarks.